The ASCO Annual Meeting 2025 officially took place in Chicago from May 30 to June 3, bringing together oncologists, researchers, and advocates worldwide to spotlight the latest breakthroughs in cancer care.

ASCO 2025 featured more than 450 oral presentations across 24 Oral Abstract Sessions and 24 Rapid Oral Abstract Sessions, 13 Clinical Science Symposia, 16 interactive Case-Based Panels, and collaborative joint sessions with AACR and ESMO. Over 2,700 posters were displayed throughout the meeting, including many from the Trials in Progress track, offering a comprehensive look at innovation across oncology.
This year’s theme, “Driving Knowledge to Action: Building a Better Future,” set a powerful tone focused on translating research into patient impact. The opening day launched with packed scientific sessions, compelling ASCO Voices presentations, and the energy of thousands united by a shared mission to advance cancer care.
Results and updates were shared for several pivotal clinical trials, including the DESTINY-Breast09, SOHO-01, NIAGARA, KRYSTAL-7, SERENA-6, MATTERHORN, CheckMate 214, SURE-02, CheckMate 901, ATOMIC, BREAKWATER, INAVO120, KEYNOTE-630, and STELLAR-002. (For the other trials, visit OncoDaily’s Oncolibrary section.)
On May 30th, the OncoDaily Party 2025 was also held, featuring the presentation of the Yvonne Awards, which recognized outstanding contributions in categories such as Breakthrough Research, Global Oncology, Community Oncology, Leadership, Mentorship, Challenging the Status Quo, Pediatric Oncology, and the Humanitarian Award.
With five packed days of science and storytelling, ASCO 2025 delivered a powerful platform for knowledge exchange and collaboration. If you missed anything, we’ve got you covered.
Our team at OncoDaily has curated 20 must-see highlights from ASCO 2025. Scroll down to explore the science, revisit standout moments, and hear from the voices shaping the future of cancer care.
“Today marks the official end of my term as ASCO President. It’s been a profound honor serving in this role. Thank you to our global ASCO community for your engagement, collaboration, and commitment to our mission of conquering cancer through research, education, and promotion of the highest quality patient care.
A message for us all: As we return home from ASCO25 armed with new knowledge and renewed energy, let’s remember we have the power to drive understanding to action and build a brighter future for ALL! My full President’s Address is available here.
A heartfelt thank you to the ASCO25 chairs: Dr. Erika P. Hamilton, Scientific Program Committee chair, and Dr. Cardinale B. Smith, Education Program Committee chair. These two women are stellar leaders and have devoted massive amounts of time and energy to develop a fabulous program for us all. Finally, sincere congratulations to Dr. Eric Small as he begins his term as 2025-2026 ASCO President. I look forward to your leadership!”
“Deeply honored to step into the role of 2025-26 ASCO President.
My focus will be guided by the theme, ‘The Science and Practice of Translation: Improving Cancer Outcomes Worldwide.’ Looking forward to a productive and collaborative year ahead with our incredible ASCO community!”

“AI is a hot topic in all fields, and oncology is no exception. For me, the question isn’t if cancer care providers will use AI. It’s about the speed at which AI will become so seamlessly integrated into our practices that we aren’t even always aware of its presence. This matters now because oncology professionals face many challenges that require fast, reliable access to trusted and timely information, and AI is how we will meet this moment.
To support our community in this effort, the American Society of Clinical Oncology (ASCO) recently announced a powerful collaboration with Google Cloud that will help oncology professionals get and stay ahead with AI. Our AI-based ASCO Guidelines Assistant combines our respective expertise to provide clinicians with fast, interactive access to ASCO’s full library of evidence-based information, so they can spend more time with patients and less time searching for reliable answers.
Physicians must always be responsible for clinical decision-making, but our Guidelines Assistant should help them quickly get to the specific, accurate, and trustworthy information they need to review to optimally treat their patients. And physician feedback is also vital, especially in these early days. Please use the “thumbs up” and “thumbs down” buttons and share your input so our team can continually improve the tool.
It was a pleasure discussing ASCO Guidelines Assistant with Google Cloud CEO Thomas Kurian during an ASCO25 session on AI, collaboration, and the future of cancer care.
In my career, I can’t recall another time when technology held so much promise and potential to transform cancer care. I’m proud that ASCO is supporting our members this way, and I’m excited for what’s ahead as we pursue the most innovative and effective ways to conquer cancer. Check out the ASCO Guidelines Assistant here.”

“Another annual meeting of the American Society of Clinical Oncology has come to an end. Chicago, once again, feels empty.
On May 30, during the Yvonne Awards by OncoDaily, our version of the Oscars in oncology. I had initially planned a long speech. But since I was the last to speak and the only thing standing between the people and the dance floor was me, I decided to keep it short.
It was 2013. We had just landed in Chicago. I was a third-year clinical fellow and a recipient of ASCO’s IDEA award. We were welcomed by two Vanessas and Kim, who later became dear friends. Twenty young doctors from developing countries, all attending the world’s largest oncology gathering for the first time. Each of us was handed an envelope, our 10-day stipend, which covered some gifts for back home, daily essentials, and a pair of brown shoes I bought on sale at a nearby Macy’s.
Years went by. A few days ago, on May 30, when I glanced out from the stage during the Yvonne Awards ceremony, I saw the leaders of the oncology world from all corners of the globe, and I saw young doctors, just like we once were, experiencing their very first ASCO whirlwind. And through the lights pouring in from the windows behind them, I saw a reflection of a younger me, arriving in the big city from a distant land, proudly wearing his new brown shoes.
The Yvonne Awards and the OncoDaily Party have, without a doubt, become major events. Friday night at ASCO, under the lights of Chicago. It’s all the result of a dream, of hard work by a dedicated team—our OncoDaily family. Proof that everything is possible, that challenges only shape us, and that the best is still ahead.
But none of this would be real without the people, those who quietly help at every step, with kind words and kind actions, with smiles, and with the genuine joy of celebrating others, whether friends or strangers. These are the people who make the world better. And it was pure happiness to be surrounded by them that night, and every day at ASCO.
Yes, it’s all about people.”

“Excited to kick off the ASCO 2025 meeting with a dynamic session on AI in cancer care!
During this opening session, we explored how ASCO is actively integrating AI into their guidelines with guideline assist and how practices are using decision-support tools to enhance patient outcomes. We discussed the transformative role of AI in clinical research, enabling faster, more precise insights that can drive innovation.
Key highlights included:
- ASCO’s initiatives to incorporate AI into clinical guidelines and tools
- The pivotal role of AI in advancing clinical research and personalized medicine
- Models of nudges and clinical decision support systems to promote evidence-based care
- Practical approaches and pearls for oncology leaders as they incorporate AI into everyday practice
As oncology continues to evolve, leveraging AI thoughtfully can truly revolutionize patient care. Looking forward to continued collaboration and innovation in this exciting space! Great to work with Douglas Flora and Ravi B. Parikh on this session!”

American Society of Clinical Oncology:
“‘Together, we are defining cancer care for generations to come with hope fueled by the many successes we achieve together at ASCO, with hope from the enduring commitment, courage, and passion from our volunteers and members, and with hope from the communities we have built along the way.’
View the ASCO25 President’s Address delivered by 2024-2025 ASCO president Dr. Robin Zon, addressing technology, advocacy, and community in cancer care.”

“Today at ASCO25 and simultaneously published in NEJM Group, Pfizer shared pivotal overall survival and progression-free survival data for a targeted treatment regimen in patients with metastatic Colorectal Cancer (mCRC) with a BRAF V600E mutation. Read more here.
For about 1 in 5 people diagnosed with CRC, the disease has already metastasized by the time it’s detected, making treatment more complex.
The BREAKWATER study met its primary endpoint of progression-free survival, as well as a key secondary endpoint of overall survival, showing that the regimen halved the risk of death for people with mCRC with a BRAF V600E mutation. These exciting results support its potential to be a new standard-of-care and a meaningful advance for these patients.”

“We had an excellent conversation on early-onset cancers in our session today at ASCO 2025.
So many important issues were raised by the audience, making sure our definitions of early onset and AYA are more inclusive for people who fit into both, that fertility discussions are far too low in the clinic, that stigma keeps young people from talking about their cancer and that we need to engage primary care providers to ensure red flag symptoms are noted and diagnosis is not delayed.
We really need to address early-onset cancer on a national level.
Cancer scientists need much more data and to hear from many more people affected by early-onset cancers to learn how best to reverse their course.
Let’s keep the conversation going!
Registrants can check out the recording using the link.”

European Institute of Oncology:
“For the first time in the history of the ASCO (American Society of Clinical Oncology) congress, two speakers from the same center have been selected for the prestigious “Oral Breast Session” dedicated to breast cancer: they are Giuseppe Curigliano, Deputy Scientific Director and Director of New Drug Development for Innovative Therapies IEO, and Paola Zagami, a young researcher from the same Division who is also awarded the illustrious ASCO Merit Award for the innovative nature of her research.
To find out about the innovations presented in therapies for both early and metastatic breast cancer, read the press release on the IEO website.”

Memorial Sloan Kettering Cancer Center:
“ICYMI from ASCO25: Yelena Janjigian shared research from a clinical trial that demonstrated a powerful new approach to help prevent cancer from coming back after treatment for stomach cancer and cancer of the lower portion of the esophagus and #esophagealjunction.
Learn more about the research here.”

“Kicking off ASCO 2025 with GI Oncology Highlights!
Day 1 delivered some standout insights, starting with a case-based panel on ctDNA testing and surveillance in colorectal cancers.
ctDNA is proving to be a highly prognostic tool, especially for detecting molecular recurrence. There’s growing interest in its potential to guide early interventions and improve short-term outcomes, though its impact on long-term survival remains uncertain.
The afternoon featured two pivotal phase III trials in metastatic colorectal cancer:
- BREAKWATER Study:
Encorafenib + Cetuximab + mFOLFOX6 for BRAF V600E-mutant mCRC showed a PFS of 12.8 months vs. 7.1 with standard care, and OS nearly doubled (30.3 vs. 15.1 months). - CheckMate-8HW (MSI-H/dMMR mCRC):
First-line Nivolumab + Ipilimumab showed an astonishing PFS of 54.1 months vs. 5.9 months with chemo.
Personalized treatment is rapidly becoming the standard, not the exception. On to Day 2!”

“It’s Day 1 of ASCO2025, and the session on financial toxicity has left me deeply reflective.
As oncologists, we frequently discuss side effects like fatigue, nausea, alopecia, and neutropenia. However, we talk far less about the invisible burden that gradually erodes dignity and security: Financial Toxicity (FT).
Coined in 2013, FT refers to the negative financial and psychological impact of cancer-related expenses. I’ve witnessed its toll in every health system I’ve worked in, which manifests in various ways:
- Loss of income due to time off work
- Cost of travel for treatment
- Paying for childcare during appointments
- Out-of-pocket costs for wigs, bras, and prescriptions
- Parking fees at hospitals, yes, patients still have to pay to park while fighting for their lives.
The emotional toll can also be significant, leading to guilt, anxiety, treatment delays, and even non-adherence.
Breast cancer patients are especially vulnerable due to:
- Higher caregiver burdens
- Lower average income
- Multimodal, prolonged treatments
- Often experience abandonment or divorce after their diagnosis
Recently, one of my patients returned to work mid-treatment, not because she was ready but because she couldn’t afford to miss rent. Patients are forced to choose between health vs survival, treatment vs food, life vs living.
In Europe, the UK has the second-lowest paid sick leave period. Our systems need urgent reform.
I must also raise an uncomfortable question: Are we, as healthcare professionals, contributing to financial toxicity?
- When we ignore the costs of supportive meds
- When we prescribe beyond third-line treatments without questioning their real-world value
- When we fail to ask, ‘Can this patient afford this choice?’
Doctors are trained to heal, not to discuss finances.
I regularly refer patients with brain metastases for stereotactic radiosurgery (SRS) in Sheffield. However, we need to consider the additional costs for the patients and their caregivers (accommodation, meals, transportation, and logistics) that they wouldn’t incur if they were local to Leicester. We risk adding unseen burdens to already heavy shoulders.
I’ve had patients unable to pick up their medications due to work commitments or because they couldn’t afford transportation.
What can we do?
- Start the conversation: ‘Is cost a concern for you?’
- Eliminate avoidable costs: No one should pay for parking during cancer treatment.t
- Arrange for pharmacies to deliver medications when possible
- Evaluate treatments beyond the third line for meaningful benefits
- Provide financial navigators to help patients access support
- Advocate for affordable and accessible treatments
- Push for systemic change
- Incorporate economic burden into the medical curriculum
We must be proactive, not reactive. A cancer diagnosis should never lead to financial ruin.”

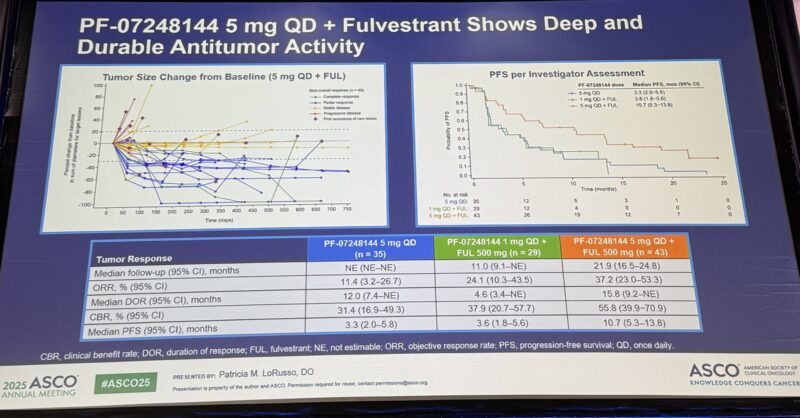
“Very promising data with KAT6 inhibition + fulvestrant presented by Pat LoRusso.
Among 43 patients with HR+/HER2- MBC, all with prior CDK4/6i, ORR was 37%, mPFS 10.7 months. Main toxicity: 74% with dysgeusia (paging Antonio Giordano, for the pronunciation of this toxicity).”
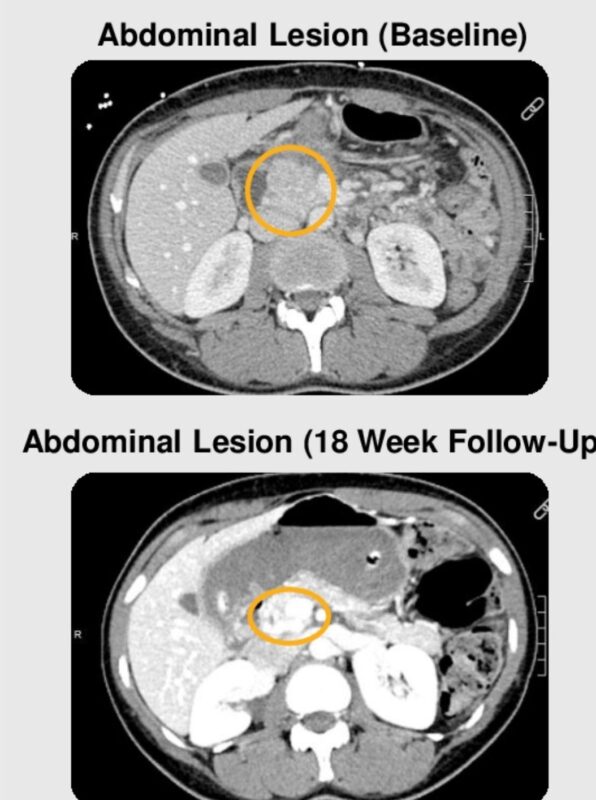
“ASCO25 looks at some of these responses!
These are patients with MSS(‘cold tumors’), not the MSI-High(‘hot tumors’). This is a big unmet need in colorectal cancer and other cancers.
- Vilastobart (XTX101)
- Tumor-activated CTLA4
- +PDL1-
Work led by Dr. Marwan Fakih.”
“On Day 2 of ASCO2025, ECO President Csaba Dégi and colleagues met with representatives from the Ukrainian Ministry of Health and Medical Professionals to learn more about the status of cancer care during this time of conflict.
Key discussion points included:
- Ukraine’s integration into international oncology education and leadership training.
- Thanks to the participation of 8 Ukrainian cancer centres in the INTERACT EUROPE 100 project, more than 100 early-career cancer professionals in Ukraine will benefit from the project’s state-of-the-art interspecialty course.
- Gaps in cancer screening and delayed diagnoses due to the war.
This exchange underscores the importance of international collaboration in safeguarding the rights and well-being of both cancer patients and professionals in Ukraine.
We’re committed to ensuring these voices are heard and supported through global platforms like ASCO.
Stay connected via the ECO Emergencies and Crises Network.
INTERACT-EUROPE 100 is co-funded by the European Union and is part of Europe’s Beating Cancer Plan.”

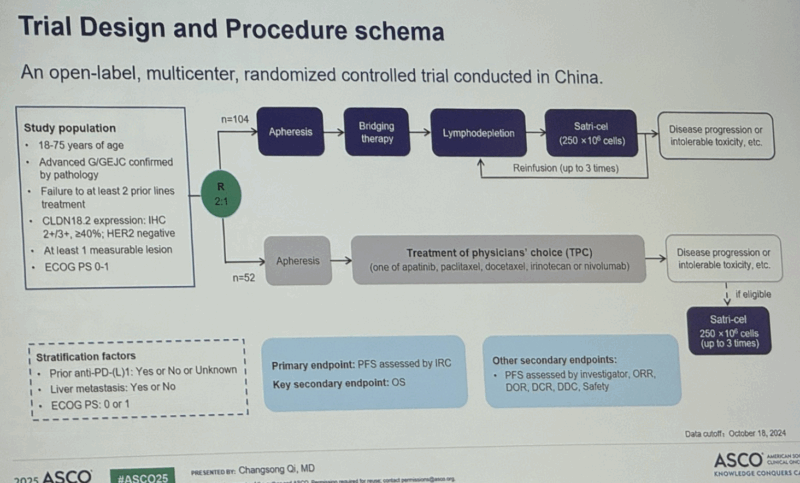
“First trial of CAR-T cell treatment for solid tumor response.
Satricabtagene autoleucel (satri-cel)/CT041 demonstrated significant progression-free survival (PFS) improvement and a clinically meaningful overall survival (OS) benefit in patients with previously treated, advanced G/GEJC.
Globally, this is the first ever randomized controlled trial of a CAR T-cell therapy in solid tumors to achieve superiority.
Satri-cel showed a manageable safety profile consistent with previous phase I results.
This trial expanded the percentage of CLDN18.2-positive patients with G/GEJC. These results support satri-cel as a new treatment option for advanced G/GEJC.”
“5 year results from KEYNOTE-564: Adjuvant pembrolizumab in ccRCC
- DFS not reached vs 68.3 mo → HR 0.71 (95% CI: 0.59–0.86),
- OS HR 0.66 (95% CI: 0.48–0.90), median OS not reached in both arms,
- 5-year DFS rate: 60.9% (pembro) vs 52.2% (placebo),
- 5-year OS rate: 87.7% vs 82.3%,
- Benefit confirmed across subgroups: intermediate-high, high risk, M1 NED, and sarcomatoid.
- No new serious treatment-related AEs in over 3 years.”

“Major news in oncology + AI.
ASCO has just partnered with Google Cloud’s Gemini to launch the ASCO Guidelines Assistant, a bold, timely move to bridge the gap between clinical knowledge and real-world practice through advanced AI.
This AI-powered tool delivers fast, evidence-based answers to clinicians by drawing exclusively from ASCO’s own guidelines, with transparent citations and built-in interactive querying. Designed to support (not replace) clinical reasoning, it’s a milestone in evidence-based digital transformation.
- ASCO-Google Cloud Official Release
- ASCO Daily News Article
- Further Details: AI Integration
- AI in Oncology Commentary – ASCO Connection
As someone deeply involved in global oncology and AI for cancer care, I see three critical implications:
Workflow-first AI
Success in oncology AI won’t be defined by novelty, but by usability. Tools like this must become cognitive extenders, not passive search engines. Real-world validation and implementation science will be essential.
Global equity opportunity
In LMICs and underserved settings, where oncologists face extreme patient loads and limited resources, AI-powered guideline tools could democratize expert-level support. But deployment must be multilingual, context-sensitive, and equitably governed.
Next frontier: personalization
To truly transform care, these tools must evolve to integrate live patient data, trial eligibility engines, and federated learning. That’s how we bridge guidelines and individualized precision care — without compromising evidence.
Kudos to ASCO for this strategic step at the intersection of technology, knowledge, and impact. We need more bold, clinician-centered, and globally conscious AI in oncology.
Let’s ensure that what we build serves patients, supports providers, and strengthens systems everywhere.
What are your thoughts on this shift?
How do you see AI transforming oncology practice in your context?
Let’s build the future – wisely.”

“New Study at ASCO 2025.
Can large language models like GPT-4 and Claude Opus reason like oncologists?
The way we’re currently evaluating large language models, with those shiny journal titles touting multiple-choice exam benchmarks for accuracy, is just horribly WRONG.
Would you trust a fresh-out-of-med-school doctor to treat your cancer based solely on passing a multiple-choice test, without any real-world experience handling complex cases with multiple, difficult treatment options?
In our study at ASCO 2025, we assessed large language models using multiple-choice questions, but we focused on their clinical reasoning, not just their accuracy. And the results? Shocking.
We benchmarked the clinical reasoning of AI models using 273 breast oncology multiple-choice questions from the ASCO QBank.
Key findings:
GPT-4 and Claude Opus both started with high accuracy (81.3% and 79.5%, respectively).
After applying chain-of-thought prompting to simulate stepwise reasoning, Claude’s performance improved to 86.4%.
GPT-4’s accuracy slightly declined to 80.95%.
Common AI errors included. That’s where we looked at their clinical reasoning!
- Reliance on outdated guidelines
- Misinterpretation of clinical trial data
- Lack of individualized/multidisciplinary care reasoning
Conclusion: LLMs are promising tools, but still fall short in nuanced, real-world oncology decision-making. Human supervision remains essential.
“Day 4: 2.06 – ASCO 2025 – Breast Cancer Clinical Advances
Another pivotal day from Chicago with practice-changing data across the breast cancer continuum. Here are the key clinical insights:
HER2+ METASTATIC BREAKTHROUGH
DESTINY-Breast09 delivers the ultimate game-changer: T-DXd + pertuzumab vs THP in first-line HER2+ mBC achieved stunning 40.7 vs 26.9 months PFS (HR 0.56, p<0.00001). With 85% ORR and 39-month DOR, CRR 15%!
Attending T-DXd arm alone: practice-changing.
DE-ESCALATION STRATEGIES VALIDATED
neoCARHP: THP proved non-inferior to TCbHP (64% vs 66% pCR, p=0.0089) with major toxicity reduction – grade 3-4 AEs dropped from 35% to 21%. Carboplatin can be safely omitted with dual HER2 blockade.
WSG pooled analysis: 12-week HER2 blockade ± chemotherapy achieved excellent 5-year survival (97-98% OS). Patients with pCR after chemotherapy-free regimens showed outstanding outcomes.
PRECISION MEDICINE ADVANCES
CompassHER2: HER2DX genomic test effectively predicts pCR across ER subtypes – high score achieved 68% vs 19% low score (p<0.001). Precision neoadjuvant selection is here.
I-SPY2: ctDNA post-neoadjuvant chemotherapy predicts nodal burden – ctDNA(-) patients: 67% ypN0 vs 33% ctDNA(+). This could guide axillary surgery decisions.
TECHNICAL and SURGICAL INNOVATIONS
AXSANA study: In 2,596 cN+ patients, probe-guided markers (radioactive/magnetic seeds) achieved 96-100% target lymph node detection vs 90% with clips. Technical precision matters.
ENDOCRINE THERAPY UPDATES
ASTRRA: 10-year follow-up confirms OFS benefits – TAM+OFS improved DFS (84% vs 76%, HR 0.68). High-risk patients aged 40-45 showed 11% BCFI improvement.
OASIS 4: Elinzanetant provides the first approved option for AET-related hot flashes, reducing VMS by 3.5 episodes/day vs placebo (p<0.0001). Fast onset, well-tolerated.
CLINICAL IMPLICATIONS
Today’s data reinforce the evolution toward precision medicine, thoughtful de-escalation, and patient-centered care. From revolutionary metastatic treatments to optimized surgical approaches, we’re witnessing the future of breast cancer management unfold.
Attached is the photo of our delegation from the Oncology Unit at Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma (Director Prof. G. Tortora).”

See also:
- 15 Posts Not to Miss from the 1st Day of ASCO 2025
- 15 Posts Not to Miss from the 2nd Day of ASCO 2025
- 15 Posts Not to Miss from the 3rd Day of ASCO 2025
- 15 Posts Not to Miss from the 4th Day of ASCO 2025
Written by Vahe Grigoryan



