At ASCO 2025, Dr. Pasi A. Jänne and a global consortium of investigators presented new efficacy and survival data from the Phase II KRYSTAL-7 trial, the largest study to date evaluating adagrasib (ADA), a selective KRASG12C inhibitor, in combination with the PD-1 inhibitor pembrolizumab (PEMBRO) as first-line treatment for patients with advanced or metastatic KRASG12C-mutated non-small cell lung cancer (NSCLC). Importantly, results were reported across all PD-L1 expression levels, marking the first disclosure of overall survival (OS) data in this setting.
What Is the KRYSTAL-7 Trial?
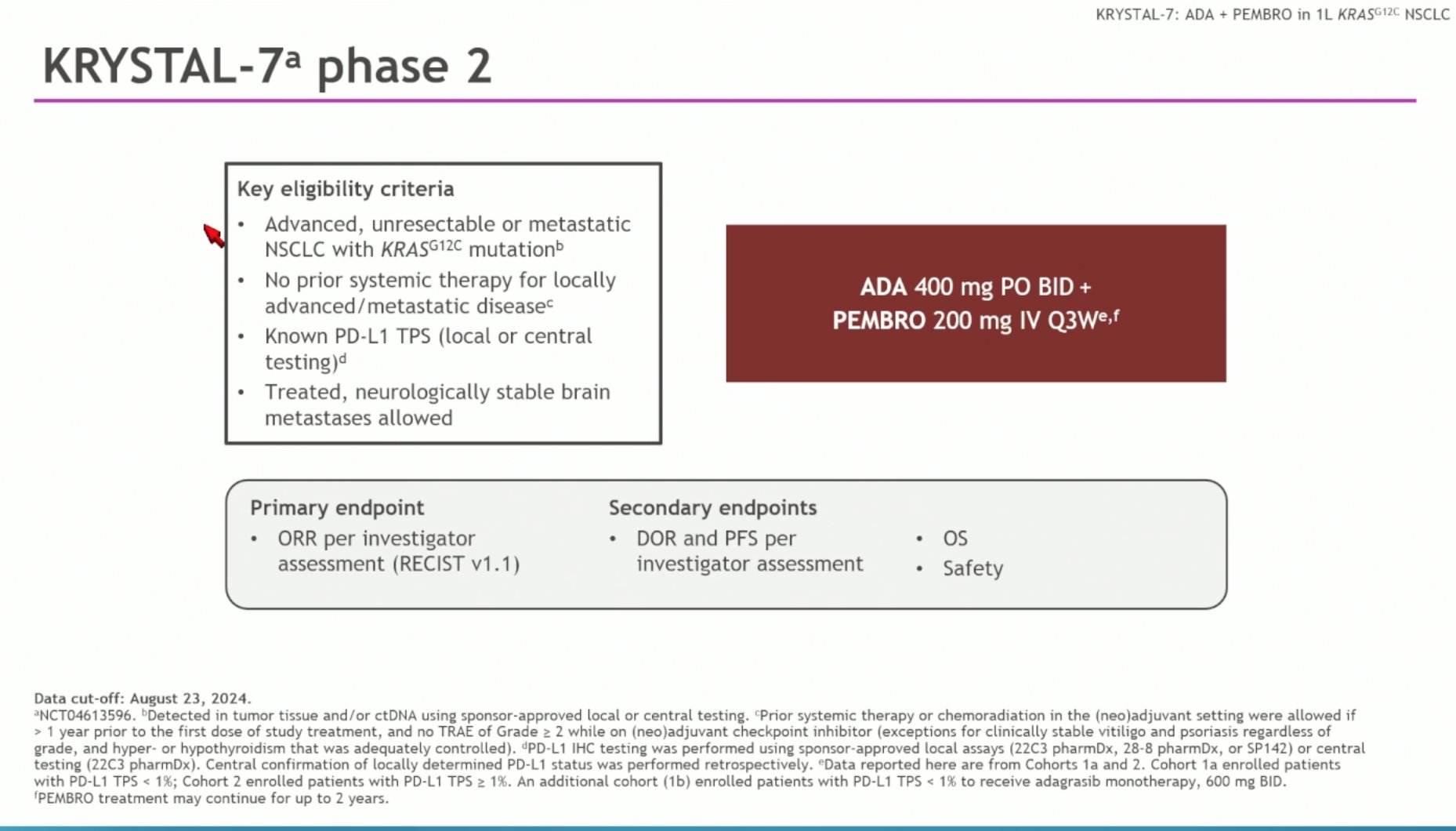
KRYSTAL-7 (NCT04613596) is a multicenter, Phase II trial investigating the combination of adagrasib 400 mg twice daily (BID) and pembrolizumab 200 mg every 3 weeks (Q3W) as first-line therapy in patients with KRASG12C-mutated NSCLC. While prior analyses focused on patients with high PD-L1 expression (≥50%), this updated report includes outcomes across all PD-L1 subgroups.
-
Primary endpoint: Objective response rate (ORR) per RECIST v1.1
-
Secondary endpoints: Duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety
Key Findings
As of August 23, 2024, the KRYSTAL-7 trial had enrolled and treated 149 patients with a combination of adagrasib (a KRASG12C inhibitor) and pembrolizumab as first-line therapy for KRASG12C-mutated advanced NSCLC. The median follow-up for overall survival (OS) at the time of analysis was 22.8 months.
The median age of participants was 67 years, with 48% female, and 62% had an ECOG performance status of 1, indicating a generally functional but symptomatic population.
Overall Efficacy
Efficacy Outcomes
Across the study population, the dual regimen delivered a confirmed objective response rate (ORR) of 44.3%, with nearly half of patients responding to treatment. Responses were durable, with a median duration of response (DOR) of 26.3 months. Patients experienced a median progression-free survival (PFS) of 11.0 months, and the 18-month PFS rate stood at 37.6%. Overall survival (OS) was also encouraging, with a median OS of 18.3 months and an 18-month OS rate of 51.8%. These findings suggest that adagrasib combined with pembrolizumab can offer meaningful and long-lasting disease control in a difficult-to-treat population.
Efficacy by PD-L1 Expression
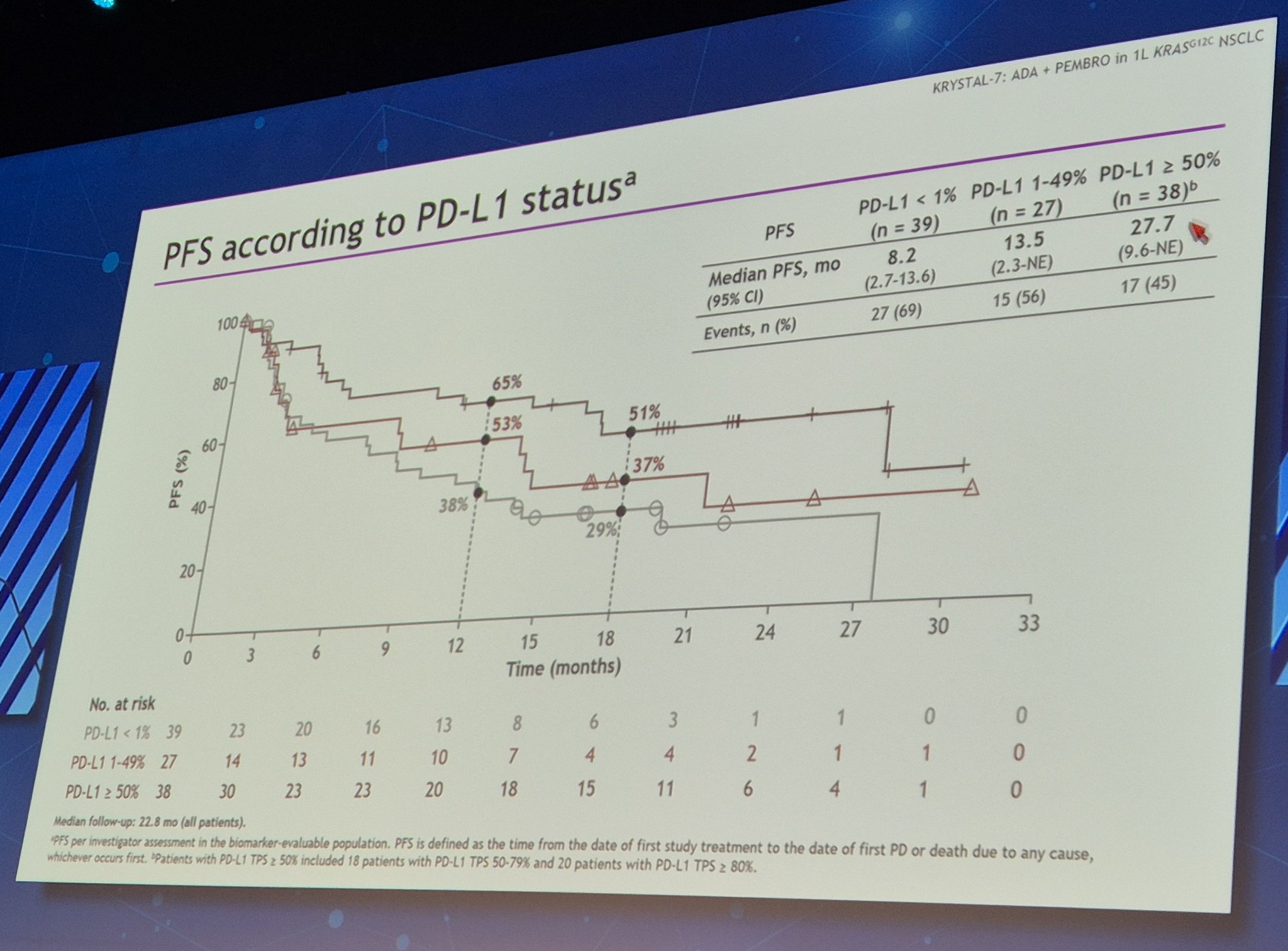
The regimen was active across all PD-L1 subgroups, but outcomes were notably stronger in patients with higher PD-L1 expression. Among patients whose tumors expressed PD-L1 at levels below 50%, the ORR was 35.8%, with a median DOR of 18.2 months and median PFS of 6.9 months. The 18-month PFS and OS rates in this group were 29.8% and 45.2%, respectively.
In contrast, patients with PD-L1 expression ≥50% showed even greater benefit. In this subgroup, the ORR rose to 59.3%, and the median DOR was 26.3 months. These patients experienced a substantial median PFS of 27.7 months, and the 18-month PFS and OS rates climbed to 50.7% and 62.4%, respectively. Median overall survival had not yet been reached in this group at the time of analysis. These results reinforce that higher PD-L1 expression may be associated with deeper and more durable responses to this combination therapy.

Safety and Tolerability
Adverse events related to treatment were common but largely manageable. Nearly 95% of patients reported at least one treatment-related adverse event (TRAE), and Grade 3 or 4 TRAEs occurred in about 68% of patients. There were three Grade 5 events (fatal): two due to pneumonia and one from pneumonitis.
Liver-related toxicities were among the most frequent adverse events. ALT elevations occurred in 39.6% of patients (with 11.4% being Grade 3/4), while AST elevations were seen in 35.6% (14.1% Grade 3/4). Alkaline phosphatase levels increased in 19.5% of patients, including 6.7% with high-grade events. Despite this, treatment discontinuations due to liver-related toxicity were relatively infrequent—only 2.0% of patients stopped adagrasib alone, 6.7% discontinued pembrolizumab, and 0.7% stopped both agents.
Overall, while low-grade liver enzyme elevations were common, they were typically manageable and transient. The safety profile remained consistent with what is expected for targeted therapy and immunotherapy combinations, supporting continued exploration of this regimen in frontline KRASG12C-mutant NSCLC.
What This Means for Patients
The KRYSTAL-7 trial represents a major advancement in the first-line treatment of KRASG12C-mutated NSCLC, a population with historically limited options. The combination of adagrasib and pembrolizumab achieved durable responses, particularly in patients with high PD-L1 expression, while maintaining a tolerable safety profile. Importantly, this is the largest dataset to date evaluating a KRASG12C inhibitor + PD-1 blockade in the first-line setting.
A Phase III portion of the study is now ongoing, comparing adagrasib + pembrolizumab versus pembrolizumab monotherapy in PD-L1 ≥50% patients, with the goal of confirming these findings in a randomized setting.
What People Are Saying About the KRYSTAL-7 Trial?
Dr. Bosch-Barrera, MD/PhD, is a thoracic oncology expert, researcher, and Assistant Professor shared on X.
“Krystal 07: Adagrasib en combinación con pembrolizumab en 1a linea de CNMP con mutacion KRAS G12C. Interesante actividad en tumores con alta expresión de PD-L1 (>50%). Habrá que esperar a resultados fase 3 randomizado, pero datos preliminares prometedores.”