The ASCO Annual Meeting 2025 is officially underway in Chicago! Running from May 30 to June 3, this year’s meeting brings together oncologists, researchers, and advocates worldwide to share the latest breakthroughs in cancer care.

ASCO 2025 is in full swing, with over 450 oral presentations delivered across 24 Oral Abstract Sessions and 24 Rapid Oral Abstract Sessions. The program also features 13 Clinical Science Symposia, 16 interactive Case-Based Panels, and collaborative joint sessions with AACR and ESMO. More than 2,700 posters, including many from the Trials in Progress track, are being showcased throughout the meeting, offering a comprehensive look at the latest advances in cancer research, clinical trials, and treatment innovation.
This year’s theme, “Driving Knowledge to Action: Building a Better Future,” sets the tone for what ASCO 2025 is all about, turning research into real impact for patients. Day 1 kicked off with a packed schedule of scientific sessions, personal stories from the ASCO Voices series, and the kind of energy you only get when thousands of people passionate about oncology come together in one place. From the latest data to big-picture conversations, it’s clear this year’s meeting is all about pushing the field forward.
Our team at OncoDaily has handpicked 15 highlights from the 4th day of ASCO 2025. Scroll down to catch the highlights, dive into the science, and hear directly from the people shaping the future of cancer care.
“Today marks the official end of my term as ASCO President. It’s been a profound honor serving in this role. Thank you to our global ASCO community for your engagement, collaboration, and commitment to our mission of conquering cancer through research, education, and promotion of the highest quality patient care.
A message for us all: As we return home from ASCO25 armed with new knowledge and renewed energy, let’s remember we have the power to drive knowledge to action and build a brighter future for ALL! My full President’s Address is available here.
A heartfelt thank you to the ASCO25 chairs: Dr. Erika P. Hamilton, Scientific Program Committee chair, and Dr. Cardinale B. Smith, Education Program Committee chair. These two women are stellar leaders and have devoted massive amounts of time and energy to develop a fabulous program for us all. Finally, sincere congratulations to Dr. Eric Small as he begins his term as 2025-2026 ASCO President. I look forward to your leadership!”

“We had an excellent conversation on early-onset cancers in our session today at ASCO 2025.
So many important issues were raised by the audience, making sure our definitions of early onset and AYA are more inclusive for people who fit into both, that fertility discussions are far too low in the clinic, that stigma keeps young people from talking about their cancer and that we need to engage primary care providers to ensure red flag symptoms are noted and diagnosis is not delayed.
We really need to address early-onset cancer on a national level.
Cancer scientists need much more data and to hear from many more people affected by early-onset cancers to learn how best to reverse their course.
Let’s keep the conversation going!
Registrants can check out the recording using the link.”

European Institute of Oncology:
“For the first time in the history of the ASCO (American Society of Clinical Oncology) congress, two speakers from the same center have been selected for the prestigious “Oral Breast Session” dedicated to breast cancer: they are Giuseppe Curigliano, Deputy Scientific Director and Director of New Drug Development for Innovative Therapies IEO, and Paola Zagami, a young researcher from the same Division who is also awarded the illustrious ASCO Merit Award for the innovative nature of her research.
To find out about the innovations presented in therapies for both early and metastatic breast cancer, read the press release on the IEO website.”

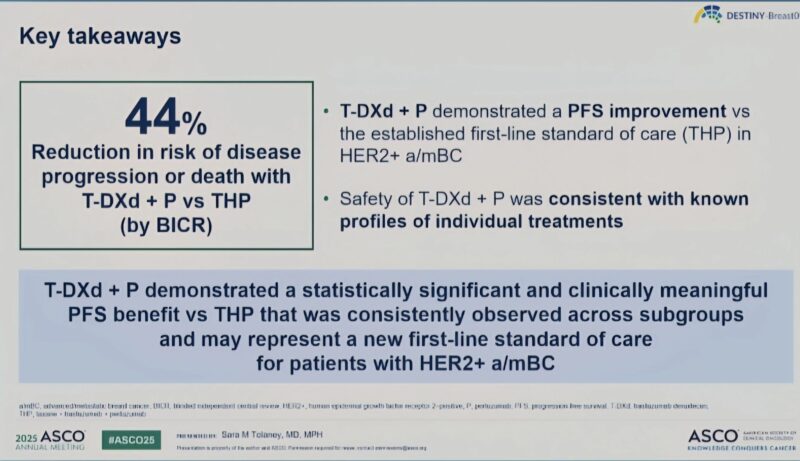
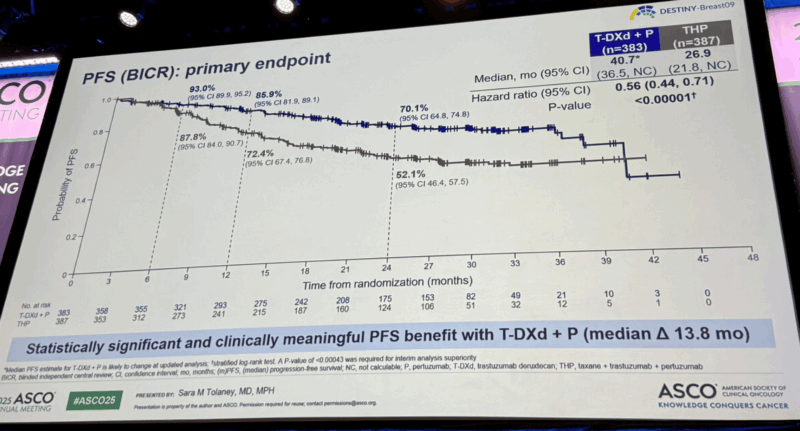
“The much-anticipated DESTINY-Breast09 (LBA1008) data was presented today by Dr. Sara Tolaney, and the results may signal a major evolution in frontline treatment for HER2+ advanced/metastatic breast cancer.
Study design:
A global phase 3 trial comparing: T-DXd (trastuzumab deruxtecan) + pertuzumab (P) vs Standard THP (taxane + trastuzumab + pertuzumab)
In treatment-naive HER2+ a/mBC patients (n=770), including those with prior neo/adjuvant therapy and disease-free interval >6 months. The T-DXd monotherapy arm remains blinded for now.
Key results at median 29-month follow-up (interim analysis):
Median PFS (BICR):
- T-DXd+P= 40.7 months
- THP-26.9 months
- HR=0.56 (P< 0.00001)
ORR: 85.1% (T-DXd+ P) vs 78.6% (THP)
CR rate: 15.1% (T-DXd +P) vs 8.5% (THP)
Median DOR: 39.2 vs 26.4 months
24-month PFS rate: 70.1% vs 52.1%
This isn’t just statistically significant, it’s clinically meaningful. A PFS of nearly 3.5 years in first-line metastatic disease is unprecedented.
But as always, we must pause and reflect:
- ILD risk remains real. Adjudicated ILD occurred in 12.1% of the T-Dxd+P group (2 grade 5 events) vs 1% with THP.
- Tolerability and sequencing must be considered. If we use our best drug first, what do we reserve for later?
- And from a patient perspective, chemo-free doesn’t mean side effect-free.
As a breast oncologist, I see the promise but also the need for balance.
We need to carefully consider who we give this regimen to in practice.
For me, these would be:
- People who progress quickly after treatment in the early setting,
- Those harbouring PIK3CA mutations,
- Those with significant visceral disease,
- Those with brain metastases, and of course, patient preference.
I also think we should consider induction T-DXd and maintenance HP in light of financial toxicity.
Bottom line: T-DXd + P significantly improves PFS vs THP and could redefine 1L therapy in HER2+ mBC. But thoughtful integration, patient selection, and long-term safety must guide how we adopt it.”
“Day 4: 2.06 – ASCO 2025 – Breast Cancer Clinical Advances
Another pivotal day from Chicago with practice-changing data across the breast cancer continuum. Here are the key clinical insights:
HER2+ METASTATIC BREAKTHROUGH
DESTINY-Breast09 delivers the ultimate game-changer: T-DXd + pertuzumab vs THP in first-line HER2+ mBC achieved stunning 40.7 vs 26.9 months PFS (HR 0.56, p<0.00001). With 85% ORR and 39-month DOR, CRR 15%!
Attending T-DXd arm alone: practice-changing.
DE-ESCALATION STRATEGIES VALIDATED
neoCARHP: THP proved non-inferior to TCbHP (64% vs 66% pCR, p=0.0089) with major toxicity reduction – grade 3-4 AEs dropped from 35% to 21%. Carboplatin can be safely omitted with dual HER2 blockade.
WSG pooled analysis: 12-week HER2 blockade ± chemotherapy achieved excellent 5-year survival (97-98% OS). Patients with pCR after chemotherapy-free regimens showed outstanding outcomes.
PRECISION MEDICINE ADVANCES
CompassHER2: HER2DX genomic test effectively predicts pCR across ER subtypes – high score achieved 68% vs 19% low score (p<0.001). Precision neoadjuvant selection is here.
I-SPY2: ctDNA post-neoadjuvant chemotherapy predicts nodal burden – ctDNA(-) patients: 67% ypN0 vs 33% ctDNA(+). This could guide axillary surgery decisions.
TECHNICAL and SURGICAL INNOVATIONS
AXSANA study: In 2,596 cN+ patients, probe-guided markers (radioactive/magnetic seeds) achieved 96-100% target lymph node detection vs 90% with clips. Technical precision matters.
ENDOCRINE THERAPY UPDATES
ASTRRA: 10-year follow-up confirms OFS benefits – TAM+OFS improved DFS (84% vs 76%, HR 0.68). High-risk patients aged 40-45 showed 11% BCFI improvement.
OASIS 4: Elinzanetant provides the first approved option for AET-related hot flashes, reducing VMS by 3.5 episodes/day vs placebo (p<0.0001). Fast onset, well-tolerated.
CLINICAL IMPLICATIONS
Today’s data reinforce the evolution toward precision medicine, thoughtful de-escalation, and patient-centered care. From revolutionary metastatic treatments to optimized surgical approaches, we’re witnessing the future of breast cancer management unfold.
Attached is the photo of our delegation from the Oncology Unit at Fondazione Policlinico Universitario A. Gemelli IRCCS Roma (Director Prof. G. Tortora).”

“Feeling incredibly honored to have had the opportunity to present at hashtag ASCO 2025 this year!
Our session, Addressing Barriers in Palliative Care for Rural and Underserved Communities, highlights the critical role community oncology practices play in bridging gaps in care and ensuring equitable access for patients facing social challenges.
It was an amazing experience to share insights alongside such a talented team of colleagues and to learn from so many inspiring leaders in the field. Events like ASCO remind me why we do what we do—advocating for patients and driving innovation in cancer care.
Thank you to everyone who attended, shared your ideas, and continues to push the boundaries of what’s possible in oncology. Let’s keep working together to make a difference!”

“Inavolisib + Palbociclib-Fulvestrant: Final OS analysis at ASCO25.
Prof. Nicholas Turner presented the final overall survival (OS) analysis of the pivotal INAVO120 Phase III trial. This study evaluated the combination of hashtag#Inavolisib (PI3K inhibitor) + Palbociclib + Fulvestrant vs. placebo + Palbociclib + Fulvestrant in patients with PIK3CA-mutated, HR-positive, HER2-negative, endocrine-resistant advanced breast cancer.
Key Takeaways:
- OS Improvement with Inavolisib + Palbociclib-Fulvestrant: The combination significantly improved OS compared to the placebo arm.
- First-ever OS improvement with PI3K Inhibition: This is the first time a PI3K pathway-targeted drug has demonstrated significant OS benefit in this patient population, marking a breakthrough for endocrine-resistant HR+, HER2- breast cancer.
- Sustained progression-free survival PFS benefit: The previously reported PFS benefit was maintained with extended follow-up, and the median time to subsequent chemotherapy was delayed by 2 years.
- Safety profile: Targeting the target is effective and safe.
Looking Ahead:
We are now eagerly awaiting the results from the ongoing GeparPiPPa study, a randomized Phase II trial that explores neoadjuvant endocrine therapy combined with Trastuzumab, Pertuzumab +/- Inavolisib in HER2-positive, HR-positive, PIK3CA-mutated primary breast cancer. This study, a collaboration between GBG Forschungs GmbH, ETOP IBCSG Partners Foundation, and AGO-B, is investigating a highly promising approach in the HER2-positive space with a focus on PI3K inhibition.”
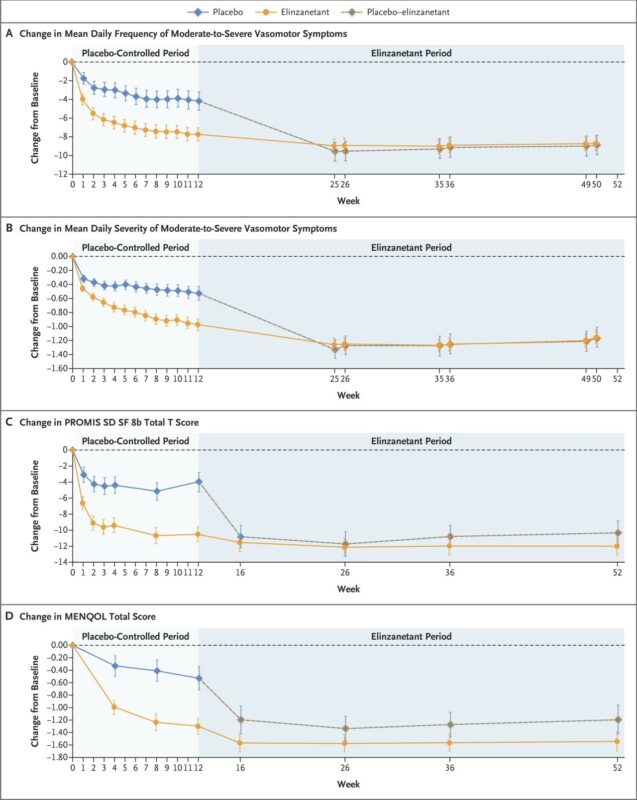
“Elinzanetant significantly reduces vasomotor symptoms in women receiving endocrine therapy for HR+ breast cancer!
In the phase 3 OASIS-4 trial (NEJM, June 2025), elinzanetant, a dual NK-1/NK-3 antagonist, led to:
- 7.8/day hot flashes at 12 weeks vs −4.2 with placebo
- >70% had ≥50% reduction
- Improved sleep and menopause-related QoL
- No hepatotoxicity signal up to 52 weeks
This could be a turning point in supportive care for breast cancer patients, who often lack safe options for menopausal symptoms. By improving the tolerability of endocrine therapy, elinzanetant may indirectly enhance long-term adherence and survival!”
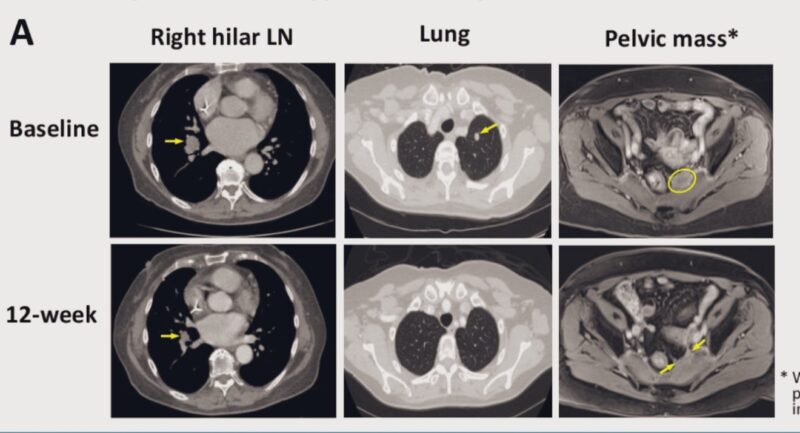
“Continuing with the immunotherapy promise for the new subset of patients with NO active LIVER METASTASES➖NLM MSS (cold )colorectal cancer. Look at these results:
- ADG126 Masked CTLA4 inhibitor + Pembro,
- ORR ~ 29%. Sustained,
- FRUQ + Immunotherapy arm.”
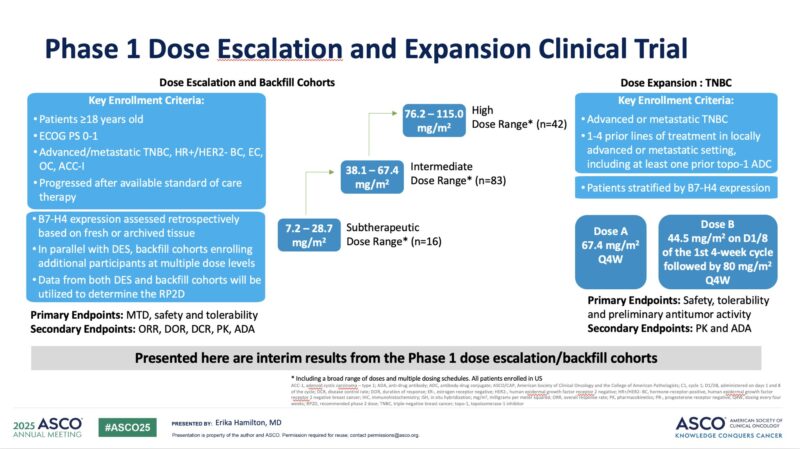
“Emiltatug-ledadotin, B7-H4 ADC, auristatin DAR 6, dose escalation, showed activity in TNBC previously treated with Topo-1 ADC, and ACC-I.
- TNBC ORR 23% (3/13)
- ORR is increased with high B7-H4
- ACC-I ORR 56% (u+cPR)
Trial expanding in TNBC 1- 4L post ADC-top1.”
“One of the things I love about ASCO annual meetings like ASCO25 is the opportunity in a short time to connect between academic disciplines and with industry to accelerate progress to help patients.”

“Long-term outcomes and OS for zanidatamab + CTx in HER2+ mGEA: 4-year follow-up. Phase-2.
- ORR 76 %m DoR 18.7 months
- mPFS 12.5 months
- mOS 36 months
- Manageable safety
- 95% concordance btw ctDNA and IHC
Strong efficacy signal, phase-3 data awaited.”
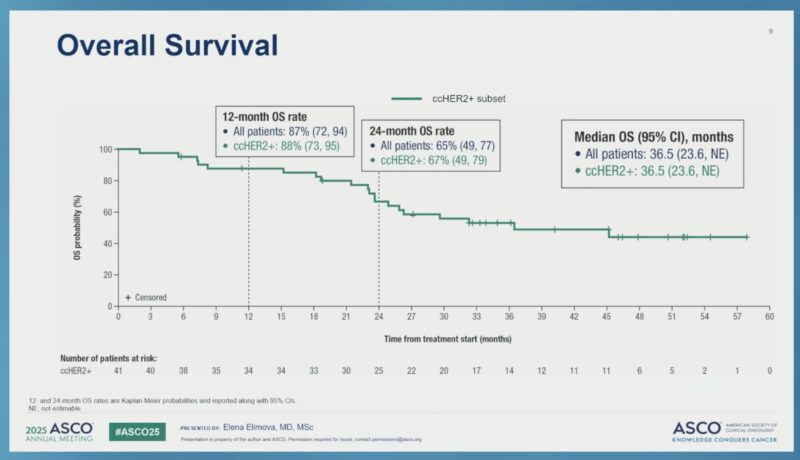
“Final OS from CheckMate 816 in resectable NSCLC at ASCO25.
- NIVO + chemo vs chemo alone (neoadjuvant),
- OS HR 0.66 → 3-yr OS: 80.2% vs 73.4%,
- pCR ↑, EFS benefit maintained,
- Benefits across subgroups,
- Only <50% of the control arm received IO at progression? “
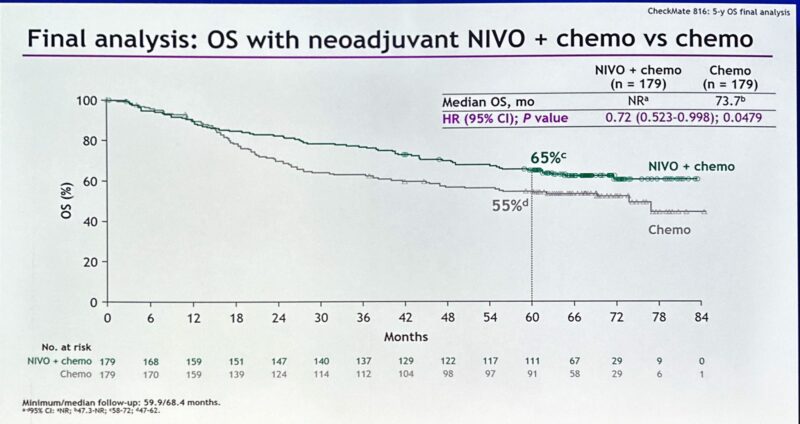
“After 30 years of failed trials, tarlatamab changes SoC for our pts with relapsed SCLC:
- mOS: 13.6 vs 8.3 months, HR=0.6, p<0.001
- Improved QoL (dyspnea and cough)
- 27% vs 62% G3 TRAEs
Grateful to all collaborators, the Amgen science team, and above all, our patients.”
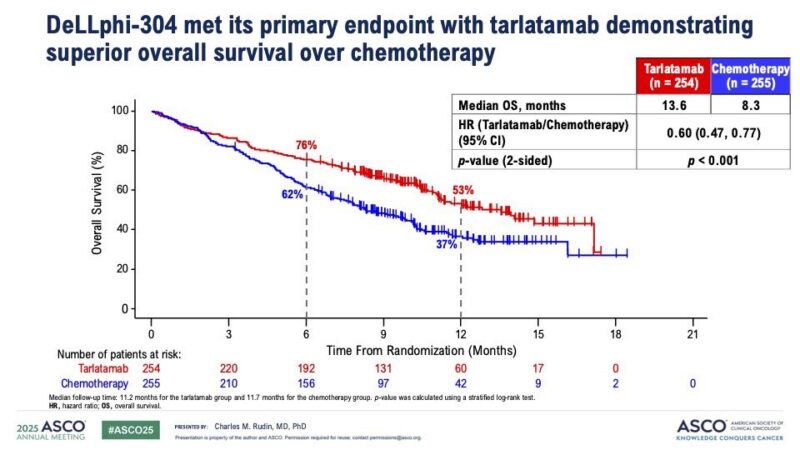
“The curves everybody’s been waiting for.
First-line T-DXd + pertuzumab significantly prolonged PFS over THP for HER2+ MBC (40.7 vs 26.9 months, HR 0.56, p<0.001), doubled the rate of complete responses (15% vs 8%), and had a positive OS trend (HR 0.84). Practice changing data.”
To stay on top of everything happening at ASCO 2025, keep following OncoDaily.
See also:
- 15 Posts Not to Miss from the 1st Day of ASCO 2025
- 15 Posts Not to Miss from the 2nd Day of ASCO 2025
- 15 Posts Not to Miss from the 3rd Day of ASCO 2025
Written by Vahe Grigoryan


