The INAVO120 phase III trial (NCT04191499) evaluated INAVO (a potent and selective PI3Kα inhibitor that also degrades mutated p110α) combined with palbociclib (PALBO) and fulvestrant (FULV) versus placebo plus PALBO and FULV in patients with PIK3CA-mutated, hormone receptor-positive (HR+), HER2-negative advanced breast cancer (aBC) resistant to endocrine therapy. The initial analysis showed a statistically significant progression-free survival (PFS) advantage with INAVO, but overall survival (OS) data were immature at that time. The current report presents the final OS results along with updated efficacy and safety data after a median follow-up of 34.2 months.

Methods
Patients received INAVO 9 mg orally once daily (Days 1–28 of each 28-day cycle) or placebo plus PALBO 125 mg orally once daily (Days 1–21 of each cycle) and FULV 500 mg intramuscularly per standard dosing. The primary endpoints included OS and objective response rate (ORR), with investigator-assessed PFS (INV-PFS) and safety as key secondary analyses.
Results
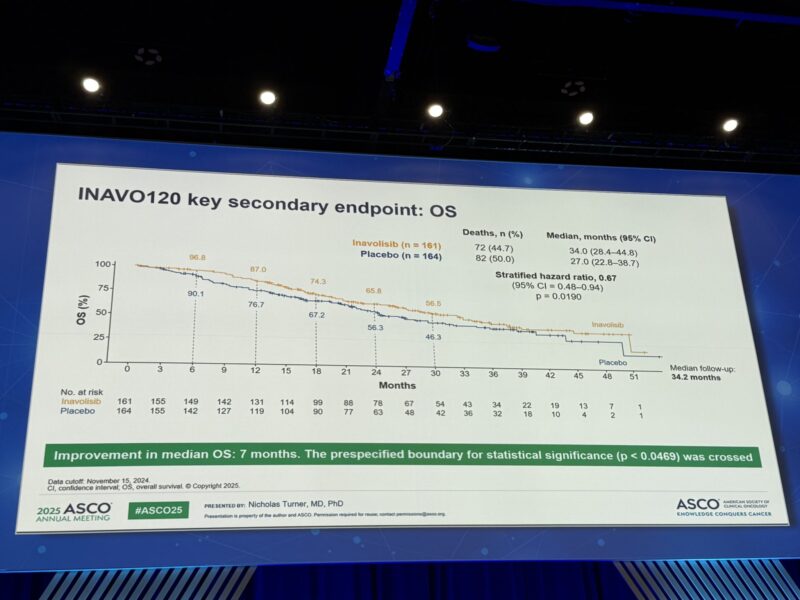
At data cutoff (November 15, 2024), median OS was significantly longer in the INAVO arm at 34.0 months (95% CI, 28.4–44.8) compared to 27.0 months (95% CI, 22.8–38.7) in the placebo arm (hazard ratio [HR] 0.67; 95% CI, 0.48–0.94; p = 0.0190), crossing the boundary for statistical significance. The OS benefit was consistent across key subgroups.
Survival probabilities at 6, 12, 18, 24, and 30 months were higher in the INAVO arm (96.8%, 87.0%, 74.3%, 65.8%, and 56.5%, respectively) than in the placebo arm (90.1%, 76.7%, 67.2%, 56.3%, and 46.3%). The ORR was markedly improved with INAVO (62.7% vs. 28.0%; p < 0.0001).
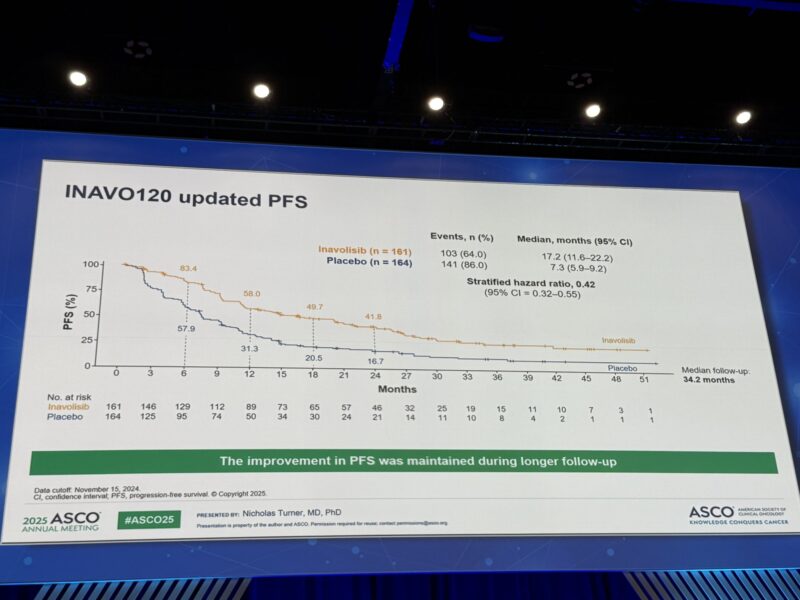
Median time to chemotherapy (TTC) was substantially delayed in the INAVO arm at 35.6 months versus 12.6 months in the placebo group (HR 0.43). Updated median INV-PFS also favored INAVO, with 17.2 months versus 7.3 months in placebo (HR 0.42), supporting a durable clinical benefit.
Regarding safety, grade 3/4 adverse events (AEs) occurred in 90.7% of patients on INAVO and 84.7% on placebo. Hyperglycemia of any grade was more frequent with INAVO (63.4% vs. 13.5%). No new grade 5 AEs were reported, and discontinuations due to AEs were low (6.8% INAVO vs. 0.6% placebo), indicating manageable toxicity.
Conclusions
The final results from INAVO120 demonstrate that adding INAVO to PALBO + FULV provides a statistically significant and clinically meaningful overall survival advantage for patients with PIK3CA-mutated, HR+/HER2– advanced breast cancer resistant to endocrine therapy. This regimen also significantly delays progression and the need for chemotherapy, with a tolerable safety profile over extended treatment.
What They’re Saying: Reactions to INAVO120 Trial at ASCO 2025
Paolo Tarantino, MD, PhD, Medical Oncologist, Clinical Fellow from DanaFarber shared on X
Nick Turner presents OS results from INAVO120. Adding inavo to 1L fulv/palvo for high-risk PIK3CAm HR+/HER2- MBC improved PFS (17 vs 7 m) & OS (34 vs 27 mo), though low crossover to alpelisib (10%). Toxicities non-negligible.
Tamar Esakia Medical Oncologist in Todua Clinic, Tbilisi shared on X
INAVO120 update: Inavolisib + palbo+ ET vs Placebo + Palbo + ET in PIK3CA-mutated, HR+, HER2- aBC PFS 17.2 vs 7.3, OS 34 vs 27, Time to CT 35,6 vs 12,6
Takeaway Messages from the INAVO120 Trial
Trial Objective: INAVO120 evaluated the efficacy of INAVO (PI3Kα inhibitor) combined with palbociclib (PALBO) and fulvestrant (FULV) in patients with PIK3CA-mutated, HR+, HER2-negative advanced breast cancer resistant to endocrine therapy.
Significant Overall Survival Improvement: The trial demonstrated a significant improvement in median overall survival (OS):
- INAVO arm: 34.0 months
- Placebo arm: 27.0 months
- HR: 0.67 (p=0.0190), confirming clinical benefit.
Durable Progression-Free Survival: Extended follow-up confirmed sustained progression-free survival (PFS):
- INAVO arm: 17.2 months
- Placebo arm: 7.3 months
- HR: 0.42, strongly favoring INAVO.
High Objective Response Rates (ORR): Patients treated with INAVO achieved more than double the ORR compared to placebo (62.7% vs. 28.0%, p<0.0001).
Delay in Time to Chemotherapy: The addition of INAVO substantially delayed the initiation of chemotherapy by approximately two years (35.6 vs. 12.6 months), significantly enhancing patient quality of life.
Consistent Safety Profile: INAVO showed a manageable safety profile, with hyperglycemia as the main adverse event (63.4% patients), yet treatment discontinuation due to adverse events remained low (6.8%).
Clinical Implications: INAVO120 confirms that combining INAVO with PALBO and FULV provides robust clinical benefit, significantly prolongs survival, delays chemotherapy, and offers a tolerable safety profile, marking it as a meaningful treatment option for endocrine-resistant, advanced breast cancer with PIK3CA mutations.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


