The ASCO Annual Meeting 2025 is officially underway in Chicago! Running from May 30 to June 3, this year’s meeting brings together oncologists, researchers, and advocates worldwide to share the latest breakthroughs in cancer care.

ASCO 2025 is in full swing, with over 450 oral presentations delivered across 24 Oral Abstract Sessions and 24 Rapid Oral Abstract Sessions. The program also features 13 Clinical Science Symposia, 16 interactive Case-Based Panels, and collaborative joint sessions with AACR and ESMO. More than 2,700 posters, including many from the Trials in Progress track, are being showcased throughout the meeting, offering a comprehensive look at the latest advances in cancer research, clinical trials, and treatment innovation.
This year’s theme, “Driving Knowledge to Action: Building a Better Future,” sets the tone for what ASCO 2025 is all about, turning research into real impact for patients. Day 1 kicked off with a packed schedule of scientific sessions, personal stories from the ASCO Voices series, and the kind of energy you only get when thousands of people passionate about oncology come together in one place. From the latest data to big-picture conversations, it’s clear this year’s meeting is all about pushing the field forward.
Our team at OncoDaily has handpicked 15 highlights from the 3rd day of ASCO 2025. Scroll down to catch the highlights, dive into the science, and hear directly from the people shaping the future of cancer care.
American Society of Clinical Oncology:
“‘Together, we are defining cancer care for generations to come with hope fueled by the many successes we achieve together at ASCO, with hope from the enduring commitment, courage, and passion from our volunteers and members, and with hope from the communities we have built along the way.’
View the ASCO25 President’s Address delivered by 2024-2025 ASCO president Dr. Robin Zon, addressing technology, advocacy, and community in cancer care.”

“Major news in oncology + AI.
ASCO has just partnered with Google Cloud’s Gemini to launch the ASCO Guidelines Assistant, a bold, timely move to bridge the gap between clinical knowledge and real-world practice through advanced AI.
This AI-powered tool delivers fast, evidence-based answers to clinicians by drawing exclusively from ASCO’s own guidelines, with transparent citations and built-in interactive querying. Designed to support (not replace) clinical reasoning, it’s a milestone in evidence-based digital transformation.
- ASCO-Google Cloud Official Release
- ASCO Daily News Article
- Further Details: AI Integration
- AI in Oncology Commentary – ASCO Connection
As someone deeply involved in global oncology and AI for cancer care, I see three critical implications:
Workflow-first AI
Success in oncology AI won’t be defined by novelty, but by usability. Tools like this must become cognitive extenders, not passive search engines. Real-world validation and implementation science will be essential.
Global equity opportunity
In LMICs and underserved settings, where oncologists face extreme patient loads and limited resources, AI-powered guideline tools could democratize expert-level support. But deployment must be multilingual, context-sensitive, and equitably governed.
Next frontier: personalization
To truly transform care, these tools must evolve to integrate live patient data, trial eligibility engines, and federated learning. That’s how we bridge guidelines and individualized precision care — without compromising evidence.
Kudos to ASCO for this strategic step at the intersection of technology, knowledge, and impact. We need more bold, clinician-centered, and globally conscious AI in oncology.
Let’s ensure that what we build serves patients, supports providers, and strengthens systems everywhere.
What are your thoughts on this shift?
How do you see AI transforming oncology practice in your context?
Let’s build the future – wisely.”

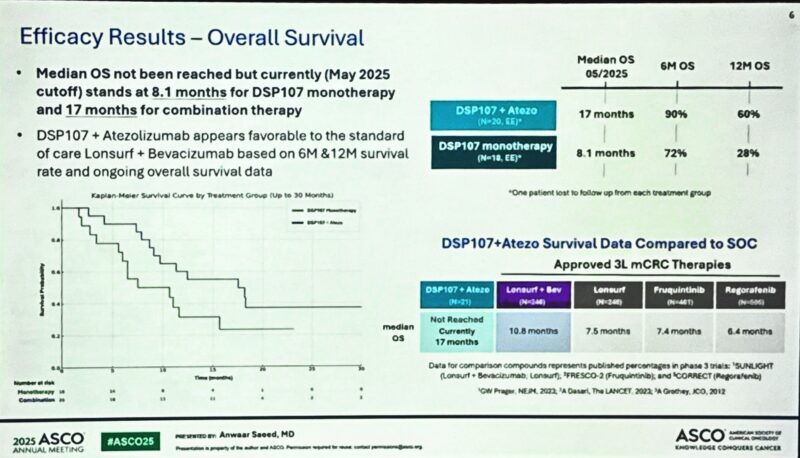
“ASCO25 DSP107 +/- Atezo in patients with MSS colorectal cancer.
Anwaar Saeed was presenting.
Interesting signal. WITH liver metastases.
CD47 increases post-FOLFOX.
Bigger unmet need for our patients with liver metastases. Something to build upon. Great work!”
“Proud moment at ASCO25!
Our poster on tumor-informed ctDNA as a predictive marker for relapse in advanced ovarian cancer drew much interest and discussion.
Findings show that ctDNA clearance at the end of chemo (C6) predicts recurrence risk better than CA-125, especially in patients with complete cytoreduction.
Amazing to connect with so many colleagues and showcase the power of Pathlight MRD technology by TeamSAGA. Thank you for the support and enthusiasm!”
“Wow! What a fantastic American Society of Clinical Oncology (ASCO) ASCO-POST editorial board meeting at ASCO25, energized by visionary leaders in oncology.
Phenomenal discussions on how to disseminate cutting-edge cancer research updates for all. Nothing beats face-to-face meetings and real-time inputs!”

The Institute of Cancer Research:
“A powerful new drug for advanced breast cancer can be used to treat emerging tumours, months before they have a chance to grow, helping to keep patients well for longer, according to new research presented today at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago.
Results of a global study, funded by AstraZeneca and co-led by researchers at The Institute of Cancer Research, London, The Royal Marsden NHS Foundation Trust and Institut Curie, Paris, showed advanced breast cancer patients who were given the new drug, called camizestrant, in combination with a CDK4/6 inhibitor cut the chances of their cancer getting worse by over half (56 per cent), allowing them to stay on first-line therapy for longer.
The SERENA-6 phase III trial is also the first global trial to demonstrate that using liquid biopsy blood tests to guide treatment at the right time, before clinical signs of progression appear, has a significant clinical benefit for this group of patients.
Co-principal investigator Professor Nick Turner, Professor of Molecular Oncology at The Institute of Cancer Research, London, and Consultant Medical Oncologist at The Royal Marsden NHS Foundation Trust, shared his thoughts.
Read the full story here.”
“Day 3: 06.01 – ASCO 2025: Key Breast Cancer Updates – Clinical Insights
Here are the clinically relevant takeaways:
PRECISION ONCOLOGY BREAKTHROUGH
SERENA-6 represents a paradigm shift: liquid biopsy-guided switching to camizestrant upon ESR1 mutation emergence nearly doubled PFS (16.0 vs 9.2 months, HR 0.44). This moves us from reactive to predictive oncology.
Angela De Michele’s plenary discussion provided exceptional clinical context – her critical analysis of trial design and real-world applicability should be required reading for practicing oncologists.
TRIPLE-NEGATIVE BREAST CANCER UPDATES
NRG-BR003: Adding carboplatin to AC→paclitaxel failed to reach statistical significance (p=0.097) despite numerical improvement in IDFS (82.9% vs 77.8%). The toxicity-benefit ratio remains unfavorable.
ABCSG 45: HRD status alone is insufficient for treatment selection. Olaparib/carboplatin achieved 77% pCR in BRCA+ patients but only 21% in BRCA-WT (vs 57% with standard TAC). BRCA mutation status remains the key biomarker.
NeoSTAR: First combination trial of sacituzumab govitecan + pembrolizumab showed 34% pCR – promising but requiring optimization of sequencing with standard chemotherapy.
CDK4/6 INHIBITOR LANDSCAPE EVOLVES
DAWNA-A: Dalpiciclib demonstrated 44% risk reduction (HR 0.56, p<0.0001) in the adjuvant setting, though a 20-month follow-up requires longer confirmation.
NATALEE subanalysis: Ribociclib benefits are consistent across all ages and menopausal status. Notably, younger premenopausal patients showed superior tolerability (10.5% vs 27.9% discontinuation in older patients).
TRADE: Game-changing dose escalation strategy for abemaciclib (50→100→150mg) achieved 71% target dose maintenance vs historical monarchE data, with only 3.4% toxicity-related discontinuation.
ADDITIONAL INSIGHTS
PROMISE: CE/BZA significantly reduced Ki-67 in ER+ DCIS (-5.6% vs -1.1%, p=0.016) without quality of life impact.
SHARE: RT APBI showed a mixed toxicity profile vs WBI – reduced skin toxicity (HR 0.55) but increased breast fibrosis (HR 2.06) and rib fractures.
CLINICAL IMPLICATIONS
These data reinforce that precision medicine in breast cancer requires biomarker-driven decisions, optimal drug sequencing, and individualized toxicity management strategies.”

“Day 3 at ASCO25: GI Oncology Shifts the Standard of Care
Two pivotal phase III trials presented today mark a turning point in the treatment of resectable gastric and stage III dMMR colon cancers.
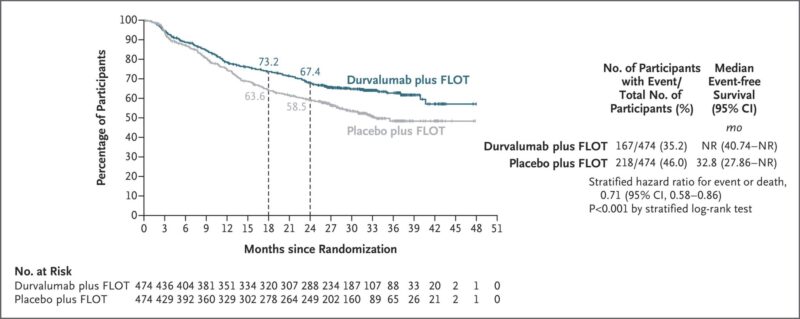
Adding durvalumab to perioperative FLOT significantly improved event-free survival (EFS) in patients with resectable gastric and GEJ adenocarcinoma.
- 2-year EFS: 67.4% vs 58.5% (HR 0.71; P<0.001)
- pCR: 19.2% vs 7.2%
Implication: This establishes durvalumab + FLOT as a new standard of care for patients with locally advanced, resectable gastric and GEJ adenocarcinoma.
ATOMIC Trial
In stage III dMMR colon cancer, adding atezolizumab to adjuvant mFOLFOX6 led to a 50% reduction in recurrence risk.
3-yr DFS: 86.4% vs 76.6% (HR 0.50; P<0.0001). Benefits are consistent across age, stage, and risk groups.
Implication: Immune checkpoint inhibitors are now a new adjuvant standard of care in dMMR colon cancer.
These studies reinforce the expanding role of immunotherapy in earlier-stage GI malignancies—beyond the metastatic setting.”
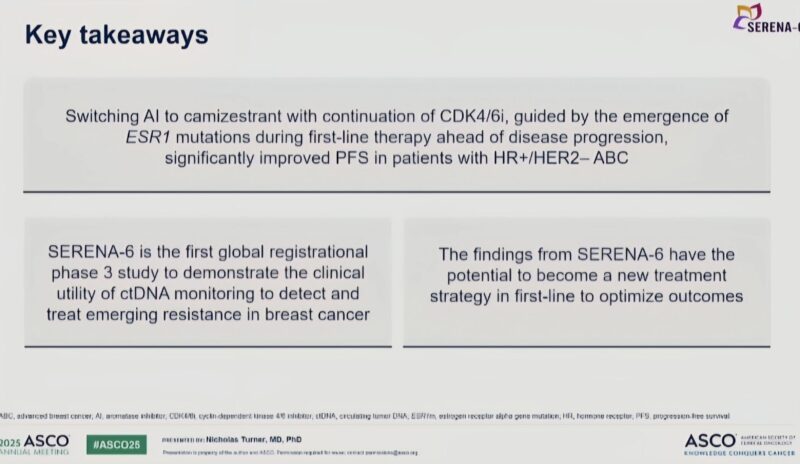
“Still Day 3 of ASCO 2025, and results from the SERENA-6 trial were presented at the plenary session.
What was studied?
This was a Phase 3, randomized trial evaluating camizestrant, a next-gen oral SERD, in patients on first-line CDK4/6 inhibitors + aromatase inhibitors (AI) with no radiographic progression but with emergent ESR1 mutations picked up through ctDNA.
Instead of waiting for clinical progression, patients were randomized to either:
- Switch AI to camizestrant + continue CDK4/6 inhibitor or
- Stay on AI + CDK4/6 inhibitor
Key findings:
- Median PFS:
Camizestrant arm = 16.0 months
AI arm = 9.2 months
(HR 0.44; P < .00001) indicating a 56% reduction in the risk of disease progression. - Time to QoL deterioration:
23.0 months vs 6.4 months (a meaningful patient-centred win)
Why does this trial matter?
Because it introduces a new way of thinking: don’t wait for progression, act on molecular signals.
ESR1 mutations are a known driver of resistance. Catching and targeting them before clinical progression may extend the benefit of first-line therapy and delay more toxic options.
Critical reflections:
- Camizestrant’s efficacy here shows promise, but what about the real-world feasibility of routine ctDNA testing? Only 17% of patients had an ESR1 mutation despite everyone having frequent blood tests.
- Is molecular progression a reliable trigger for treatment change, or are we over-medicalising without hard clinical endpoints? Patients are going to receive the drug in a later line, so should we be using this in an endocrine-sensitive population?
- With multiple oral SERDs emerging, how will we choose, especially when used pre-progression?
- From the patient’s perspective, are we adding anxiety for every blood draw to test for ESR1-mutation?
- This strategy is elegant, but cost, accessibility, and equity must be part of the rollout conversation.
Bottom line:
SERENA-6 shows that when ESR1 mutations appear, we don’t have to wait for the cancer to catch up.
Camizestrant offers a proactive, well-tolerated, and patient-friendly option that opens the door to integrating molecular surveillance into routine care.
For those of us managing HR+/HER2– advanced breast cancer, this marks a potential shift in thinking of how and when we act on resistance.”
“New Study at ASCO 2025.
Can large language models like GPT-4 and Claude Opus reason like oncologists?
The way we’re currently evaluating large language models, with those shiny journal titles touting multiple-choice exam benchmarks for accuracy, is just horribly WRONG.
Would you trust a fresh-out-of-med-school doctor to treat your cancer based solely on passing a multiple-choice test, without any real-world experience handling complex cases with multiple, difficult treatment options?
In our study at ASCO 2025, we assessed large language models using multiple-choice questions, but we focused on their clinical reasoning, not just their accuracy. And the results? Shocking.
We benchmarked the clinical reasoning of AI models using 273 breast oncology multiple-choice questions from the ASCO QBank.
Key findings:
GPT-4 and Claude Opus both started with high accuracy (81.3% and 79.5%, respectively).
After applying chain-of-thought prompting to simulate stepwise reasoning, Claude’s performance improved to 86.4%.
GPT-4’s accuracy slightly declined to 80.95%.
Common AI errors included. That’s where we looked at their clinical reasoning!
- Reliance on outdated guidelines
- Misinterpretation of clinical trial data
- Lack of individualized/multidisciplinary care reasoning
Conclusion: LLMs are promising tools, but still fall short in nuanced, real-world oncology decision-making. Human supervision remains essential.
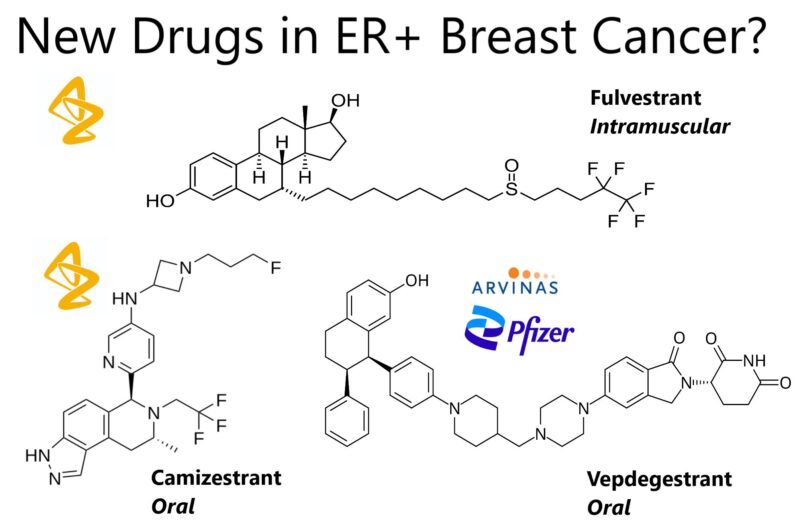
“New Winning Drugs in ER+ Breast Cancer?
The treatment landscape for advanced estrogen receptor (ER)–positive, HER2-negative breast cancer is evolving with novel oral selective estrogen receptor degraders (SERDs). Two such agents, vepdegestrant and camizestrant, have been evaluated in clinical trials, offering insights into their potential to replace the standard intramuscular SERD, fulvestrant. Hot off the press from ASCO25!
In the VERITAC-2 Phase 3 trial, published in NEJM yesterday, vepdegestrant was compared to fulvestrant in patients who had progressed on prior endocrine therapy and a CDK4/6 inhibitor. In the overall population, vepdegestrant did not significantly improve PFS (3.8 vs. 3.6 months; HR, 0.86; 95% CI, 0.70–1.06; P=0.16). However, in patients with ESR1 mutations, vepdegestrant extended median PFS to 5.0 months versus 2.0 months for fulvestrant (HR, 0.60; 95% CI, 0.43–0.83; P=0.002).
Meanwhile, camizestrant has shown broader efficacy in the SERENA-6 trial published in NEJM today. This Phase 3 study involved patients on first-line aromatase inhibitor plus CDK4/6 inhibitor therapy. ESR1 mutations were tracked via ctDNA, and patients with these mutations were randomized to switch to camizestrant plus the same CDK4/6 inhibitor or continue standard therapy. Median PFS was 16.0 months for camizestrant versus 9.2 months for continued standard therapy, a 56% reduction in risk (HR, 0.44; 95% CI, 0.32–0.60; P<0.001). Camizestrant plus CDK4/6 inhibitors was well-tolerated with low discontinuation rates.
Compared to fulvestrant, which is limited by its intramuscular administration and median PFS of 3.6 months, vepdegestrant (oral) offers targeted benefit in ESR1-mutant disease with PFS of 5.0 months. Camizestrant, also oral, demonstrated broader efficacy with a median PFS of 16.0 months in patients with ESR1 mutations detected through liquid biopsy while on first-line therapy.
Both oral SERDs represent a major advance, offering convenient administration and potential to overcome resistance mechanisms. Vepdegestrant’s activity in ESR1-mutant disease highlights its targeted promise, while camizestrant’s robust efficacy and proactive treatment strategy may establish it as a new standard of care. These findings suggest a dynamic shift in the endocrine therapy landscape, with new options poised to replace fulvestrant and improve outcomes for patients with advanced ER-positive, HER2-negative breast cancer. Really very exciting for patients! ”
“The phase 3 MATTERHORN trial shows that adding durvalumab to perioperative FLOT significantly improves outcomes in resectable gastric and gastroesophageal junction adenocarcinoma.
PD-L1 status did not significantly impact the benefit of durvalumab!
Key Findings (N=948):
- 2-year EFS: 67.4% vs 58.5% (HR 0.71; P<0.001),
- pCR rate: 19.2% vs 7.2% (RR 2.69),
- Similar Grade 3–4 AE rates: ~71% in both arms,
- No major delays in surgery or adjuvant therapy,
- Overall survival trend favored durvalumab (HR 0.67 after 12 months).”
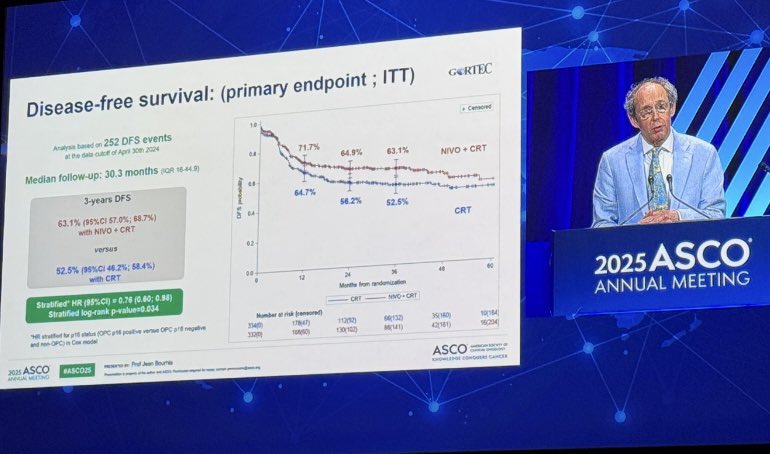
“Cisplatin + RT became the standard for high-risk LA-SCCHN and held strong for 30 years.
As a fellow, it’s meaningful to witness real change as immunotherapy is finally entering the field. A new era for HNSCC begins.”
“And of course: Lung Oral session.
My take so far:
Krystal 7 IO + adagrasib is interesting as it was tolerated, but efficacy does not seem to be better than with IO alone or with chemo. Will need to wait and see the randomized trial.
Dato shows interesting first-line data with IO.
Zipalertinib is active in EGFR ex20 (even after Ami). BAY 2927088 shows very promising activity in people with lung cancer and HER2 mutations. Savolitinib + Osi have better PFS than chemo after EGFR TKIs 1st-3rd gen) and with MET amp – new option?
Patri improved PFS marginally and is unlikely to dethrone platinum chemo after 3rd gen TKI alone. Sacituzumab improves RR, PFS, and OS v docetaxel after EGFR TKI and platinum chemo and is likely to become a preferred option.”

“Excellent multi-disciplinary panel at ASCO25 exploring PSMA PET and management of high-risk prostate cancer.
PSMA PET is 27% more accurate than conventional imaging:
- Fewer equivocal lesions (7% vs 23%)
- Management change: (28% vs 15%)
- Lower radiation dose (8.4 vs 19.2 mSv)
Surgery for cN1 disease: retrospective data suggest comparable outcomes to RT+ADT
- Better outcomes with lower ISUP grade & ≤2 pelvic LNs
- High PSA persistence in the high-risk group post-RP
Lymph node dissection trials in progress (PLND vs limited/no dissection):
- Large studies underway, including NCT03921996, NCT01812902
STAMPED: ADT+Abiraterone improves MFS (HR 0.53) in cN1/very high-risk localized disease
Selected trials in progress: PREDICT-RT, ATLAS, DASL-HiCaP, ENZARAD
- Focus on intensified RT + systemic approaches guided by Decipher, PSMA-PET
Patient-centered decision making: Balancing clinicians’ perspective with patient QoL and values.”
To stay on top of everything happening at ASCO 2025, keep following OncoDaily.
See also:
- 15 Posts Not to Miss from the 1st Day of ASCO 2025
- 15 Posts Not to Miss from the 2nd Day of ASCO 2025
Written by Vahe Grigoryan


