Standard adjuvant chemotherapy for stage III colon cancer typically involves fluoropyrimidine combined with oxaliplatin. For patients with deficient mismatch repair (dMMR), the potential benefit of integrating immune checkpoint inhibitors with adjuvant chemotherapy had remained uncertain. The ATOMIC trial (NCT02912559), a pivotal Phase III study, evaluated whether the addition of atezolizumab, an anti-PD-L1 antibody, to standard chemotherapy (modified FOLFOX6 [mFOLFOX6]) could enhance patient outcomes in surgically resected stage III dMMR colon cancer.
Methods
Sponsored by the National Cancer Institute (NCI), ATOMIC was a multicenter, randomized Phase III trial enrolling patients aged over 12 years, with surgically resected stage III dMMR colon adenocarcinoma. Tumors were centrally verified for dMMR status by immunohistochemistry. Patients (n=712) were randomized evenly to receive either:
- mFOLFOX6 chemotherapy plus atezolizumab (840 mg intravenously every 2 weeks) for 12 cycles (6 months), followed by atezolizumab monotherapy for an additional 13 cycles (totaling 12 months), or
- mFOLFOX6 chemotherapy alone for 12 cycles.
Randomization was stratified by lymph node involvement (N1/N1c vs N2), tumor invasion depth (T1–3 vs T4), and primary tumor location (proximal vs distal colon).
The primary endpoint was disease-free survival (DFS), with overall survival (OS) and adverse event (AE) profiles as secondary outcomes. DFS was evaluated using the stratified log-rank test, with hazard ratios (HRs) calculated through a stratified Cox model.

Results
Between September 2017 and January 2023, 712 patients were enrolled, median age 64 years, with 55.1% female participants. Most tumors (83.8%) originated from the proximal colon. Approximately half (46.1%) of patients had low-risk disease (T1-3N1), with the remainder categorized as high-risk (T4 and/or N2).
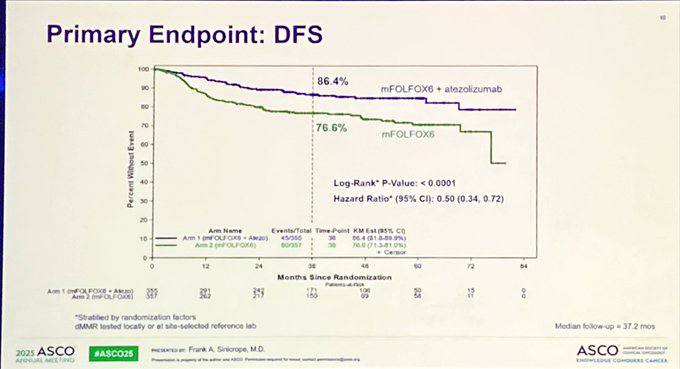
At a median follow-up of 37.2 months, 124 DFS events were observed. Three-year DFS was significantly improved in the atezolizumab plus mFOLFOX6 group compared to mFOLFOX6 alone:
- Atezolizumab + mFOLFOX6: 86.4% (95% CI, 81.8–89.9)
- mFOLFOX6 alone: 76.6% (95% CI, 71.3–81.0)
- Hazard Ratio (HR): 0.50 (95% CI, 0.35–0.72)
- p-value: <0.0001 (crossing pre-specified efficacy threshold of 0.009)
This substantial DFS improvement was consistent across all analyzed subgroups, including patients over 70 years of age and both low- and high-risk categories.
Safety Profile
Treatment-related adverse events grade ≥3 were slightly higher in the atezolizumab arm (71.7%) compared to chemotherapy alone (62.1%), though this profile was manageable and consistent with known safety profiles of immunotherapy combined with chemotherapy.
Clinical Implications
These compelling data from the ATOMIC trial strongly support the addition of atezolizumab to adjuvant chemotherapy with mFOLFOX6 as a new standard of care for patients with stage III dMMR colon cancer. This regimen offers substantial improvements in disease-free survival, representing a major advancement in adjuvant therapy for this subgroup of colon cancer patients.
Conclusion
The ATOMIC trial clearly demonstrates that adding atezolizumab to standard mFOLFOX6 chemotherapy significantly enhances DFS in patients with resected stage III dMMR colon cancer. Given the robust efficacy and manageable safety profile, this combination should now be considered the preferred adjuvant treatment approach.

Key Takeaways from the ATOMIC Trial
Landmark DFS Improvement: The addition of atezolizumab to mFOLFOX6 significantly improved 3-year disease-free survival (DFS) in patients with resected stage III dMMR colon cancer:
- Atezolizumab arm: 86.4% (95% CI, 81.8–89.9)
- mFOLFOX6 alone: 76.6% (95% CI, 71.3–81.0)
- Hazard Ratio: 0.50 (95% CI, 0.35–0.72); p < 0.0001
Statistically Significant Benefit: The trial crossed the pre-specified efficacy boundary (p < 0.009) at interim analysis with 124 DFS events and a median follow-up of 37.2 months, confirming superior efficacy of adding immunotherapy.
Broad Applicability: The benefit of atezolizumab was consistent across subgroups, including:
- Older patients (age >70)
- Both low-risk (T1–3N1) and high-risk (T4 and/or N2) tumors
Manageable Safety Profile: Grade ≥3 treatment-related adverse events:
- Atezolizumab arm: 71.7%
- mFOLFOX6 arm: 62.1%
- Most adverse events were consistent with known toxicity profiles for immunotherapy and oxaliplatin-based chemotherapy.
Clinical Impact: These findings support atezolizumab + mFOLFOX6 as a new standard of care for patients with resected stage III dMMR colon cancer, replacing chemotherapy alone in this biomarker-defined population.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


