Immunotherapy has emerged as a promising treatment option for colon cancer, particularly for patients with specific genetic markers such as microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR). This article will explore various aspects of immunotherapy for colon cancer, including the different types of treatments available, their success rates, potential side effects, and ongoing research efforts. By examining data from recent studies, we aim to provide a comprehensive overview of how immunotherapy is shaping the landscape of colon cancer treatment.
What Is Immunotherapy for Metastatic Colon Cancer
Immunotherapy is a powerful tool in treating metastatic colon cancer, which has spread beyond the colon to other parts of the body. By harnessing the body’s immune system, immunotherapy helps it recognize and fight cancer cells more effectively. Cancer cells can evade detection by using proteins like PD-L1 to “turn off” immune cells, particularly T-cells, which are vital in targeting and destroying abnormal cells.
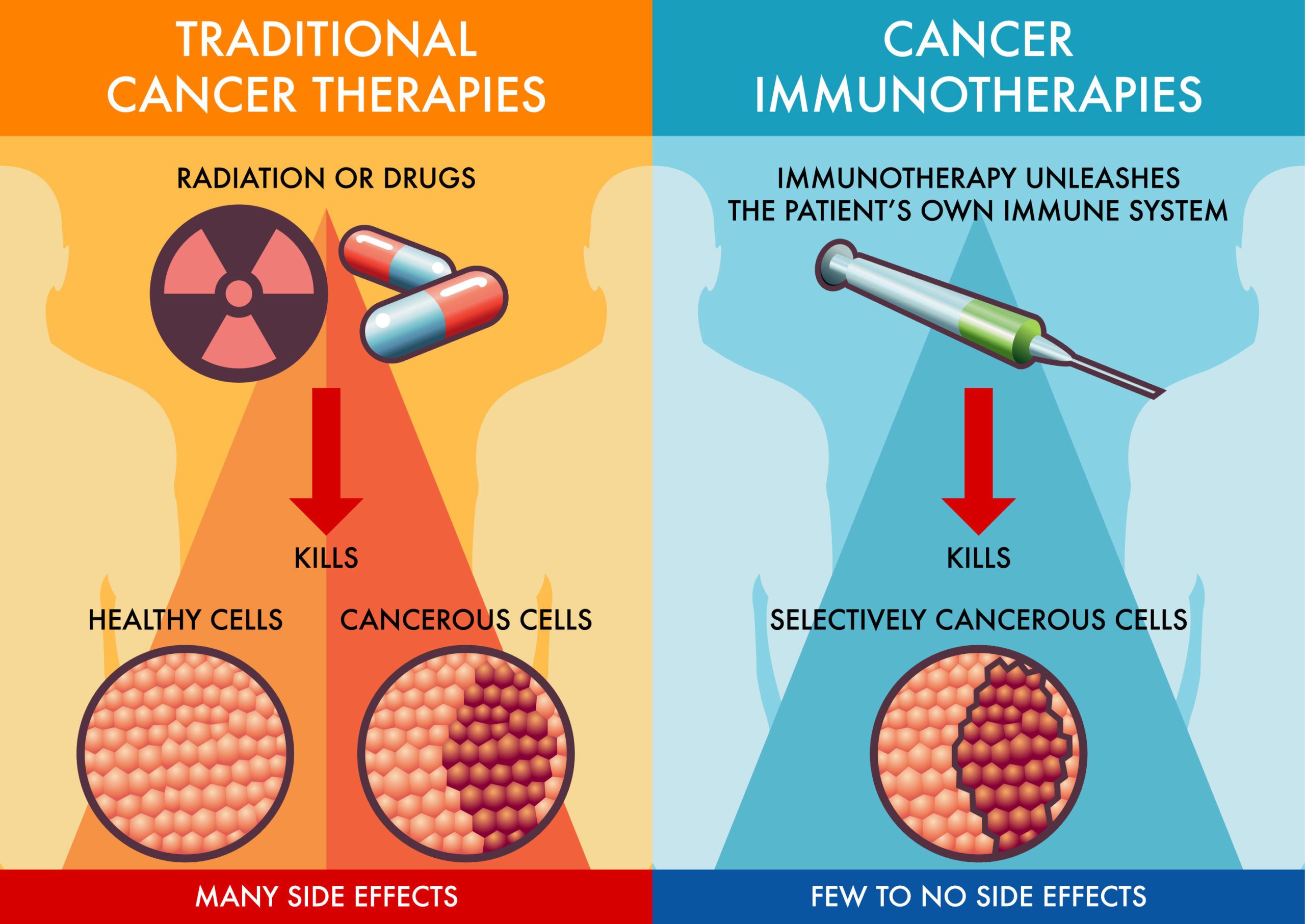
Drugs known as checkpoint inhibitors, such as pembrolizumab and nivolumab, block these proteins, allowing T-cells to stay active. This method “releases the brakes” on the immune system, enhancing its ability to detect and destroy cancer cells. These therapies are particularly effective in patients with genetic markers like MSI-H or dMMR, which make cancer cells more recognizable to the immune system.
What Are the Types of Immunotherapy for Metastatic Colon Cancer?
Immunotherapy for metastatic colon cancer includes checkpoint inhibitors, monoclonal antibodies, cancer vaccines and CAR T-cell therapy.
Checkpoint Inhibitors
Pembrolizumab (Keytruda) and nivolumab (Opdivo) are checkpoint inhibitors that target the PD-1 receptor on T-cells, preventing cancer cells from suppressing the immune response through the PD-L1 protein. By blocking this interaction, these drugs enhance the immune system’s ability to detect and attack cancer cells. In the KEYNOTE-158 trial, pembrolizumab showed an objective response rate (ORR) of 34.3% across various types of MSI-H/dMMR solid tumors, including colorectal cancer, with durable responses observed in many patients. For nivolumab, the CheckMate-142 trial demonstrated an ORR of 31% in MSI-H/dMMR metastatic colorectal cancer patients, which increased to 55% when combined with ipilimumab, as highlighted by Overman et al. in 2017.
Monoclonal Antibodies
Monoclonal antibodies like trastuzumab (Herceptin) target the HER2 protein, which is overexpressed in some types of metastatic colon cancer. By binding to HER2 receptors on cancer cells, trastuzumab blocks signals that promote tumor growth and survival, while also marking the cancer cells for destruction by the immune system. In metastatic HER2-positive colon cancer, combining trastuzumab with chemotherapy or other targeted therapies has shown improved progression-free survival and response rates. A study published in Lancet Oncology (2019) showed that adding trastuzumab to pertuzumab in HER2-positive metastatic colorectal cancer led to a 32% response rate with durable responses in a subset of patients
Cancer Vaccines
Cancer vaccines, a form of immunotherapy, are designed to stimulate the immune system to recognize and attack cancer cells by targeting tumor-associated antigens like CEA or MUC1, which are commonly overexpressed in metastatic colon cancer. Recent studies, including a 2023 phase II trial published in Nature Medicine, demonstrated that a personalized neoantigen vaccine, when combined with immune checkpoint inhibitors, significantly improved progression-free survival in patients with metastatic colorectal cancer. While still experimental, these vaccines show promise, particularly for microsatellite stable (MSS) tumors that are less responsive to standard immunotherapies. Current research is also exploring the synergistic effects of combining vaccines with other treatments, such as chemotherapy and targeted therapies, to further enhance immune activation and tumor control.
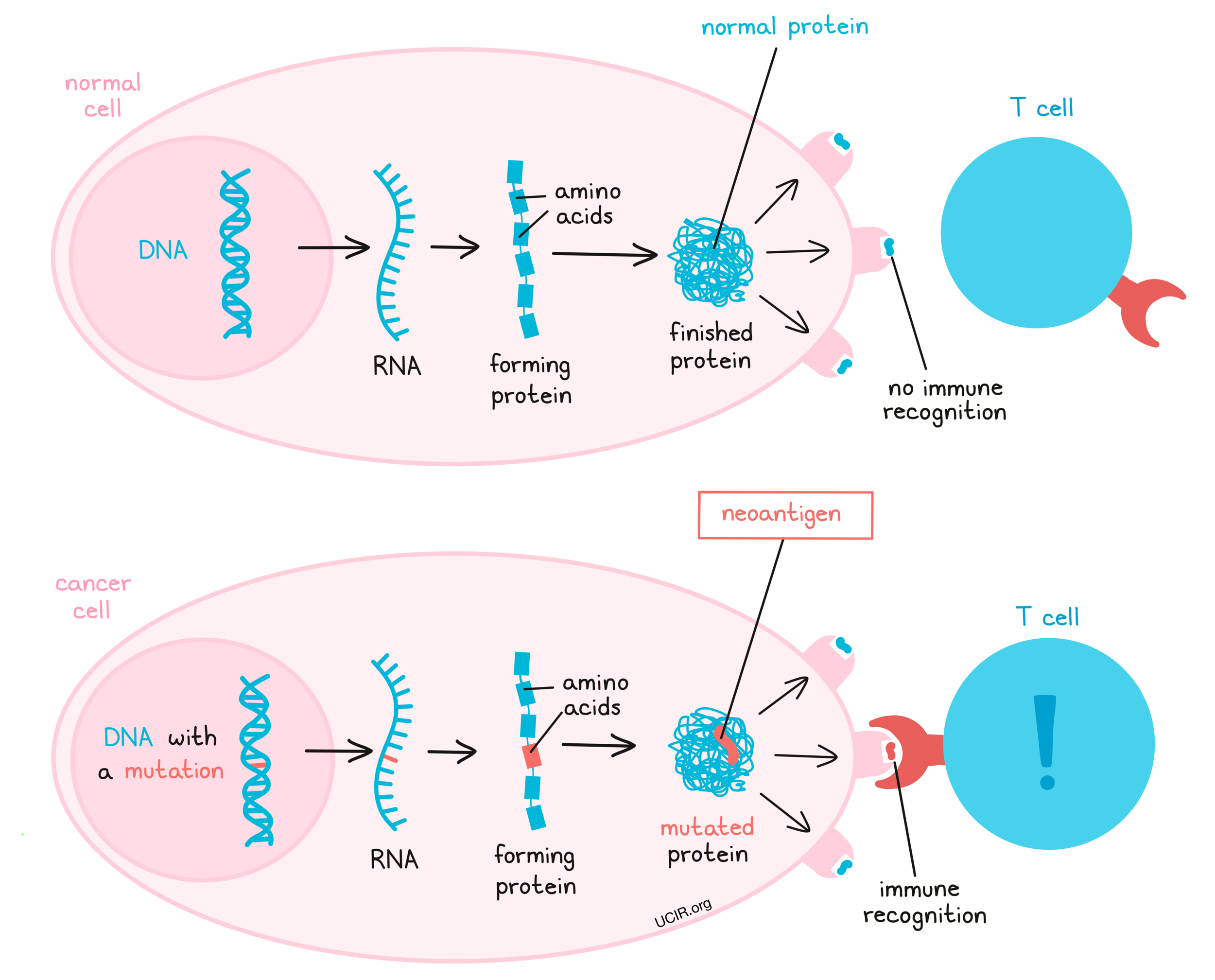
CAR T-Cell Therapy
CAR T-cell therapy modifies a patient’s T-cells to target specific proteins on cancer cells. For metastatic colon cancer, it shows potential, particularly in targeting carcinoembryonic antigen (CEA), a protein often overexpressed in colon tumors. The process involves extracting and engineering T-cells to recognize tumor-specific antigens and reinfusing them back into the patient. While CAR T-cell therapy has proven effective in blood cancers, challenges exist for solid tumors like colon cancer due to the tumor microenvironment that can suppress immune responses. Recent 2022 trials have shown promising results, with some patients experiencing partial tumor regression and disease stabilization, particularly in microsatellite stable (MSS) colon cancer cases. Continued research aims to improve its effectiveness in these challenging cases.
How Effective Is Immunotherapy for Metastatic Colon Cancer?
Immunotherapy has proven effective for patients with MSI-H metastatic colon cancer, with treatments like nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor) significantly improving outcomes. The NCT02997228 trial demonstrated a promising overall response rate of approximately 52% when using a combination of nivolumab and ipilimumab in advanced cases. Newer agents, such as botensilimab and balstilimab, are currently under investigation for their potential to further enhance immune responses, including in MSS colorectal cancer cases, which have historically shown limited responses to immunotherapy. These developments highlight the expanding role of combination immunotherapies and bring hope for broader treatment efficacy across colorectal cancer types.
Success Rates for Microsatellite Instability-High (MSI-H) Metastasis Colon Cancer
Immunotherapy has shown significant effectiveness in treating metastatic colon cancer with microsatellite instability-high (MSI-H) characteristics. Patients with MSI-H tumors are particularly responsive to immune checkpoint inhibitors, such as pembrolizumab and nivolumab, due to their unique genetic profile that makes them more recognizable to the immune system.
Studies have reported impressive outcomes for these patients. According to the KEYNOTE-177 trial, which evaluated pembrolizumab as a first-line treatment for patients with MSI-H/dMMR metastatic colorectal cancer, the objective response rate (ORR) was approximately 43.8%, with a median progression-free survival (PFS) of 16.5 months. The study also highlighted that patients treated with pembrolizumab had a higher likelihood of maintaining disease control compared to those receiving standard chemotherapy.
The durable responses observed in MSI-H metastatic colon cancer patients underscore the transformative potential of immunotherapy. Long-term follow-up studies have shown that a significant proportion of patients experience lasting remission, reinforcing the need for genetic testing to identify MSI-H tumors as candidates for immunotherapy, which could lead to improved outcomes and survival rates.
Success Rates for Microsatellite Stable (MSS) Colon Cancer
For MSS metastatic colon cancer, immunotherapy has limited success due to lower mutation burden, making tumors less recognizable to the immune system. However, new agents like botensilimab (CTLA-4 inhibitor) and balstilimab (PD-1 inhibitor) are showing promise in early trials by enhancing T-cell response in MSS tumors. These therapies aim to overcome the immune-suppressive environment of MSS cancers and provide better responses than traditional immunotherapies. Ongoing clinical trials are assessing the potential of these agents to improve survival rates and deliver more durable responses for MSS colon cancer patients.
What Are the Side Effects of Immunotherapy for Metastatic Colon Cancer?
Immunotherapy for metastatic colon cancer can result in a range of side effects that vary in severity. While some side effects are common and generally manageable, others can be serious and require close monitoring.
Common Side Effects
Patients undergoing immunotherapy often experience fatigue, which is reported by 30-50% of patients. This can be moderate to severe and may affect daily functioning. Another frequent side effect is nausea, although it is generally less intense compared to that caused by chemotherapy, with anti-nausea medications helping to manage this symptom. Skin reactions, including rashes and itching, occur in up to 40% of patients. These are typically mild and can be treated with topical creams, though in more severe cases, systemic treatment may be necessary.
Serious Side Effects
More serious side effects include autoimmune reactions, where the immune system mistakenly targets healthy organs. This can lead to complications like colitis (inflammation of the colon), hepatitis, pneumonitis, and thyroiditis, all of which require prompt medical attention. Additionally, infusion reactions may occur during treatment, presenting symptoms ranging from mild fever to severe allergic reactions, such as difficulty breathing or anaphylaxis. These rare but potentially life-threatening events underscore the importance of close monitoring and early intervention during treatment.
How Is Immunotherapy Administered?
For metastatic colon cancer, immunotherapy treatments, such as pembrolizumab or nivolumab, are typically administered via intravenous (IV) infusions. The frequency and duration of treatment depend on the patient’s response and tolerance.
Patients often receive treatment every 2 to 6 weeks, and the total treatment duration can extend up to two years, especially in cases of a good or sustained response. In cases where a patient shows a strong positive response, treatment may be paused after two years of continued success. Conversely, treatment may be stopped earlier if the patient experiences intolerance or severe side effects that outweigh the benefits.
What Should Patients Expect During Treatment?
The patient experience with immunotherapy for metastatic colon cancer starts with an initial consultation where eligibility is assessed based on factors like genetic markers and overall health. If approved, treatment involves IV infusions every 2-3 weeks, with sessions lasting 30 minutes to a few hours. Side effects such as fatigue, skin rashes, or GI issues are monitored, and supportive care, including anti-nausea meds or corticosteroids, may be provided for severe reactions. Regular imaging and blood tests every 8-12 weeks help track treatment response and adjust as needed. After treatment, follow-up care focuses on monitoring for delayed side effects and ensuring cancer control through periodic scans and check-ups.
How Long Does It Take to See Results from Immunotherapy?
Patients typically observe improvements or changes after starting immunotherapy for metastatic colon cancer within 2-3 months. In the first 8-12 weeks, doctors perform initial scans to assess tumor response. Some patients may experience pseudo-progression, where tumors appear larger before shrinking, due to immune cell activity. By 3-6 months, significant changes such as tumor shrinkage or stabilization are often confirmed. Every patient responds differently, and regular monitoring through scans helps track progress and guide further treatment decisions.
What Are the Costs of Immunotherapy for Metastatic Colon Cancer?
Immunotherapy for metastatic colon cancer can cost over $100,000 per year, with expenses varying based on treatment duration. Insurance coverage helps, but out-of-pocket costs may still be high. Financial assistance programs, such as those from Patient Access Network Foundation and CancerCare, offer support for those facing financial difficulties. Consulting healthcare providers or financial counselors can help identify options to manage costs.
How Does Immunotherapy Compare to Other Treatments for Metastatic Colon Cancer?
When comparing immunotherapy to traditional treatments like surgery, chemotherapy, and targeted therapy for metastatic colon cancer, each option presents distinct advantages and limitations.
You can read more about Immunotherapy vs Chemotherapy: Article by OncoDaily
- Immunotherapy (e.g., pembrolizumab, nivolumab) is highly effective for patients with MSI-H/dMMR tumors, offering durable responses and the potential for long-term remission. It typically has fewer severe side effects compared to chemotherapy, though it works best in specific genetic subtypes. For non-MSI-H patients, the response is limited, and it may be less effective.
- Surgery is primarily used for isolated metastases, particularly in the liver or lungs, and can significantly extend survival when combined with systemic treatments. However, surgery is rarely curative in stage 4 cases, and many patients are not candidates due to the spread of the disease.
- Chemotherapy (e.g., FOLFOX, FOLFIRI) remains a cornerstone of treatment for metastatic colon cancer, especially for MSS tumors that don’t respond to immunotherapy. It can shrink tumors and prolong survival, but often comes with more severe side effects, such as fatigue, nausea, and hair loss, and responses are generally short-term.
- Targeted therapies (e.g., bevacizumab, cetuximab) aim at specific mutations or pathways involved in tumor growth. These therapies are effective in prolonging progression-free survival but are typically limited by the development of resistance over time and are used in combination with chemotherapy for enhanced effectiveness.
What Are the Long-Term Effects of Immunotherapy?
Immunotherapy for metastatic colon cancer can offer significant long-term benefits, particularly for patients with MSI-H/dMMR tumors, leading to prolonged remission and improved survival outcomes. Many patients experience durable responses, meaning their cancer remains controlled for extended periods after treatment ends. These positive outcomes often contribute to a better quality of life compared to chemotherapy or other treatments.
However, some patients may face ongoing health challenges related to the immune system, such as chronic autoimmune conditions, including colitis, thyroid dysfunction, or lung issues like pneumonitis. Fatigue and hormonal imbalances are also common and may require ongoing medical attention. Thus, regular monitoring and follow-up care are crucial to manage these effects and ensure continued well-being.
Can All Metastatic Colon Cancer Patients Receive Immunotherapy?
Eligibility for immunotherapy in colon cancer is primarily determined by the tumor’s genetic profile and the patient’s overall health. Patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) tumors are the most likely candidates, as these cancers respond better to immune checkpoint inhibitors like pembrolizumab or nivolumab. Additionally, patients must generally have a good performance status, meaning they are physically well enough to tolerate treatment, and should not have severe autoimmune conditions, which could worsen with immunotherapy.
What Research Is Being Done on Immunotherapy for Metastatic Colon Cancer?
Current research on improving immunotherapy for metastatic colon cancer is focusing on both new drug developments and combination therapies aimed at overcoming resistance, especially in microsatellite stable (MSS) colon cancer patients, who traditionally respond poorly to standard immunotherapies.
New Drugs
Botensilimab (a CTLA-4 inhibitor) and balstilimab (a PD-1 inhibitor) are two promising investigational drugs. Early trials are showing that these agents, particularly when used together, may stimulate a more effective immune response even in MSS colon cancer, which makes up the majority of metastatic colon cancer cases. These drugs are designed to better modulate the immune system, encouraging a more robust attack on cancer cells that typically evade immune detection.

You can read more on 10 most promising cancer drugs, not yet approved: Special Article by Oncodaily
Combination Therapies for Enhanced Efficacy
Combining checkpoint inhibitors with other treatment modalities has shown particular promise in mCRC. For instance:
- Nivolumab and Ipilimumab Combination: Trials such as CheckMate 142 have demonstrated that dual therapy with nivolumab (a PD-1 inhibitor) and ipilimumab (a CTLA-4 inhibitor) can yield substantial responses. In patients with MSI-H (microsatellite instability-high) tumors, this combination therapy showed a 55% response rate with a 12-month survival rate of 85%. This strategy targets multiple immune pathways to overcome the immunosuppressive tumor environment.
- Atezolizumab in Combination: Although atezolizumab monotherapy did not yield the desired results in the IMblaze 370 trial, combination studies like AtezoTRIBE have shown that adding atezolizumab to chemotherapy and bevacizumab (a VEGF inhibitor) improved progression-free survival in MSS mCRC, achieving 13.1 months versus 11.5 months in control groups. This promising combination is under further investigation to verify its efficacy.
Personalized Approaches with Novel Biomarkers
Research is increasingly focusing on identifying biomarkers beyond MSI-H/dMMR (deficient mismatch repair) status to personalize treatments. High tumor mutational burden (TMB) is emerging as a potential indicator of a positive response to immunotherapy. Trials are assessing how TMB and other biomarkers can better guide treatment plans, potentially extending immunotherapy benefits to MSS patients who historically respond poorly.
Future Directions and Novel Targets
The landscape of immunotherapy for metastatic colon cancer is evolving with continuous clinical trials exploring novel agents and targets:
- KRAS G12C Inhibitors: Targeting KRAS mutations, especially the G12C variant, offers hope for patients with this mutation. Investigational agents such as sotorasib and adagrasib are being tested with positive early results, demonstrating that KRAS-targeted therapy may be feasible for some mCRC patients.
- HER2-Targeted Therapies: For HER2-positive mCRC, combinations like tucatinib and trastuzumab are delivering promising response rates, as seen in the MOUNTAINEER trial, where a 38.1% objective response rate was observed. These therapies provide an option for the subset of patients with HER2 amplification.
As these studies progress, the goal is to translate clinical trial success into broader, accessible treatment strategies that offer improved survival and quality of life for metastatic colorectal cancer patients. With ongoing trials validating these approaches, the future of immunotherapy in mCRC looks promising for both MSI-H and MSS patients.
You can watch more about novel KRAS G12C drug Glecirasib: Interview by Oncodaily
How Can Patients Support Their Immune System During Treatment?
To support the immune system during immunotherapy, patients may benefit from several lifestyle changes.
- Dietary Adjustments: Focus on a balanced diet rich in fruits, vegetables, whole grains, and lean proteins to provide essential nutrients that support immune health. Foods high in antioxidants, such as berries and leafy greens, help reduce inflammation.
- Regular Exercise: Moderate physical activity, like walking or yoga, can improve immune function and help manage fatigue.
- Hydration: Drink plenty of water to maintain hydration, which supports overall body function and helps manage side effects.
- Supplements: Some patients consider vitamin D and probiotics to boost immune function, but it’s crucial to consult with a doctor before starting any supplements to avoid interference with treatment.
- Stress Management: Techniques like meditation or deep breathing can reduce stress, which is known to negatively impact immune health.
Always consult your healthcare provider before making significant lifestyle or dietary changes during treatment.
More on Immunotherapy for All stages of Colon Cancer: Article by Oncodaily
FAQ
What Is Immunotherapy for Stage IV Colon Cancer?
Immunotherapy for stage IV colon cancer uses drugs to stimulate the immune system to target and destroy cancer cells. It is most effective in tumors with specific genetic markers, like MSI-H or dMMR, which make cancer cells more detectable to immune cells.
Can Stage IV Colon Cancer Be Cured with Immunotherapy?
While immunotherapy can lead to long-term remission in some cases of stage IV colon cancer, a complete cure remains challenging. Success largely depends on the tumor’s genetic profile, with better outcomes in MSI-H or dMMR cancers.
What Are the Side Effects of Immunotherapy for Colon Cancer?
Common side effects of immunotherapy include fatigue, skin rash, and mild nausea. Serious effects can include autoimmune reactions, such as colitis or pneumonitis. Each patient’s experience may vary, so monitoring by healthcare providers is essential.
Who Is Eligible for Immunotherapy in Stage IV Colon Cancer?
Patients with MSI-H or dMMR genetic markers are the best candidates for immunotherapy in metastatic colon cancer. However, eligibility also depends on overall health and tolerance for potential side effects.
How Long Does It Take to See Results from Immunotherapy?
Results from immunotherapy may be observed within 8-12 weeks. Scans are typically done every few months to evaluate treatment effectiveness, and some patients may experience temporary tumor growth before seeing a reduction in size.
Is Immunotherapy Covered by Insurance for Stage IV Colon Cancer?
Insurance often covers immunotherapy, though out-of-pocket costs can still be significant. Many programs provide financial assistance for eligible patients to help with the high costs of treatment.
How Does Immunotherapy Compare to Chemotherapy for Stage IV Colon Cancer?
Immunotherapy generally has fewer severe side effects than chemotherapy, but its effectiveness is limited to certain genetic subtypes like MSI-H or dMMR. Chemotherapy remains the primary treatment for other types of metastatic colon cancer.
Are There Any Experimental Immunotherapies for Stage IV Colon Cancer?
Yes, new drugs like botensilimab and balstilimab are currently being tested, especially for microsatellite-stable (MSS) cancers. Clinical trials are exploring how these can improve immune response in cases that don’t typically respond to standard immunotherapy.
What Support Options Exist for Managing Side Effects During Immunotherapy?
Patients can manage side effects through lifestyle adjustments, like staying hydrated, eating a balanced diet, and practicing stress-reducing activities. Health providers may also prescribe medications to alleviate specific symptoms, such as nausea or skin rash.
Can Immunotherapy Be Used with Other Treatments for Better Results?
Yes, immunotherapy is often combined with chemotherapy, targeted therapy, or other drugs to improve its effectiveness, especially in challenging cases like MSS colon cancer.
Written by Toma Oganezova, MD