The ASCO Annual Meeting 2025 is officially underway in Chicago! Running from May 30 to June 3, this year’s meeting brings together oncologists, researchers, and advocates worldwide to share the latest breakthroughs in cancer care.

ASCO 2025 is in full swing, with over 450 oral presentations delivered across 24 Oral Abstract Sessions and 24 Rapid Oral Abstract Sessions. The program also features 13 Clinical Science Symposia, 16 interactive Case-Based Panels, and collaborative joint sessions with AACR and ESMO. More than 2,700 posters, including many from the Trials in Progress track, are being showcased throughout the meeting, offering a comprehensive look at the latest advances in cancer research, clinical trials, and treatment innovation.
This year’s theme, “Driving Knowledge to Action: Building a Better Future,” sets the tone for what ASCO 2025 is all about, turning research into real impact for patients. Day 1 kicked off with a packed schedule of scientific sessions, personal stories from the ASCO Voices series, and the kind of energy you only get when thousands of people passionate about oncology come together in one place. From the latest data to big-picture conversations, it’s clear this year’s meeting is all about pushing the field forward.
Our team at OncoDaily has handpicked 15 highlights from the 2nd day of ASCO 2025. Scroll down to catch the highlights, dive into the science, and hear directly from the people shaping the future of cancer care.
“Day 2 of ASCO 2025.
Today’s oral abstract presentation of ASCENT-04/KEYNOTE-D19 (LBA109) brought game-changing data and real hope for patients with PD-L1+ metastatic triple-negative breast cancer (TNBC).
This Phase 3 trial compared sacituzumab govitecan (SG) + pembrolizumab vs chemo + pembrolizumab in previously untreated, PD-L1+ (CPS ≥10) locally advanced/metastatic TNBC.
And the results?
- Median PFS:
SG + pembro = 11.2 months
Chemo + pembro = 7.8 months
(HR 0.65; P = .0009) - Median duration of response (DOR):
SG + pembro = 16.5 months
Chemo + pembro = 9.2 months - ORR:
59.7% (SG + pembro) vs 53.2% (chemo + pembro) - Fewer treatment discontinuations due to side effects with SG + pembro (12% vs 31%)
Even though overall survival (OS) data is still maturing, there is already a positive early trend.
For me, as a breast oncologist, this trial marks a pivotal shift. Sacituzumab govitecan, already proven in heavily treated TNBC, is now showing power upfront, in combination with immunotherapy.
Here’s what stands out:
- Durability of response is impressive.
- Better tolerability profile than standard chemo combos.
- Meaningful impact on quality of life by delaying progression and reducing discontinuation rates.
It also reaffirms a vital point: we must design more trials that challenge conventional first-line choices. In a setting like TNBC that is historically so unforgiving, patients deserve better than “chemo-first” by default. Approximately 50% of patients do not receive second-line treatment. 34% die before second-line treatment.
While we await OS data, this combination is already reshaping the treatment landscape for PD-L1+ TNBC.
The path forward must include access, equity, and real-world validation, but the science is encouraging.
Well done to the ASCENT-04 team (Sara Tolaney et al). This data should redefine the standard of care.”

“Huge congratulations to Heidi Klepin of our Associate Director of the Atrium Health Wake Forest Baptist Comprehensive Cancer Center, for the ASCO25 B.J. Kennedy Geriatric Oncology Award for a career filled with compassion and impact for the elderly facing cancer!”

“It’s only Saturday of ASCO25, and my heart is full!
Thank you, American Society of Clinical Oncology (ASCO), for this honor. Congrats to all awardees! And I am just so happy to be with my people! So honored to have received FASCO today, and be able to share it with so many dear colleagues and friends.
It’s an honor to serve the American Society of Clinical Oncology (ASCO) in its mission! ”

“ASCO25 looks at some of these responses!
These are patients with MSS(‘cold tumors’), not the MSI-High(‘hot tumors’). This is a big unmet need in colorectal cancer and other cancers.
- Vilastobart (XTX101)
- Tumor-activated CTLA4
- +PDL1-
Work led by Dr. Marwan Fakih.”
“On Day 2 of ASCO2025, ECO President Csaba Dégi and colleagues met with representatives from the Ukrainian Ministry of Health and Medical Professionals to learn more about the status of cancer care during this time of conflict.
Key discussion points included:
- Ukraine’s integration into international oncology education and leadership training.
- Thanks to the participation of 8 Ukrainian cancer centres in the INTERACT EUROPE 100 project, more than 100 early-career cancer professionals in Ukraine will benefit from the project’s state-of-the-art interspecialty course.
- Gaps in cancer screening and delayed diagnoses due to the war.
This exchange underscores the importance of international collaboration in safeguarding the rights and well-being of both cancer patients and professionals in Ukraine.
We’re committed to ensuring these voices are heard and supported through global platforms like ASCO.
Stay connected via the ECO Emergencies and Crises Network.
INTERACT-EUROPE 100 is co-funded by the European Union and is part of Europe’s Beating Cancer Plan.”

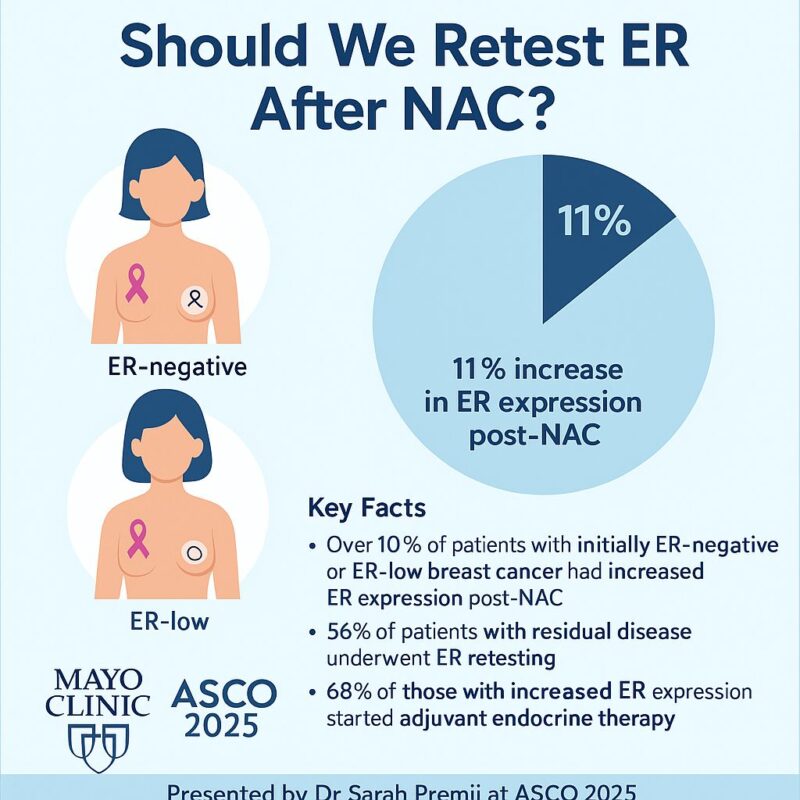
“ER Expression May Change Post-NAC. Should We Be Retesting?
New ASCO 2025 data (Abstract 529) show that >10% of patients with pretreatment ER-negative or ER-low breast cancer had increased ER expression in residual tumor tissue after neoadjuvant chemotherapy (NAC).
More than half underwent ER retesting.
Among those retested, 11% showed increased ER expression, opening the door for adjuvant endocrine therapy.
Particularly relevant for triple-negative and HER2+ cases treated as ER-negative pre-NAC.
Key Message: If there’s residual disease post-NAC, consider ER retesting to individualize adjuvant therapy.”
“Today at ASCO2025 (Day 2), the final overall survival results from the INAVO120 trial were presented and simultaneously published in the New England Journal of Medicine.
This phase III study evaluated inavolisib, a potent oral PI3Kα inhibitor, in combination with palbociclib and fulvestrant, compared to standard palbociclib + fulvestrant, in patients with PIK3CA-mutated, HR+/HER2- advanced breast cancer.
Key Findings:
- Median OS: 46.7 months (inavolisib arm) vs 39.3 months (control)
HR 0.75: a 25% reduction in the risk of death. - Median PFS: 15.0 months vs 11.2 months. HR 0.75.
- Time to Chemotherapy: Median of 36.1 months vs 25.9 months. That’s nearly a full year longer before needing chemotherapy, which truly matters at the bedside.
- Patient population: Over 80% had visceral disease. This was a group with a significant disease burden. These are the patients who need rescue early.
- Safety: Grade ≥3 neutropenia (52%), hyperglycemia (10%), rash (8%). Discontinuation due to side effects in 13.4%
What does this mean in the clinic?
As a breast oncologist, I see many younger women who progress quickly after adjuvant endocrine therapy and CDK4/6 inhibitors. They often present with visceral, life-threatening disease, and we need options that act fast and buy time.
Inavolisib shows us we can delay disease progression, defer chemotherapy, and perhaps most importantly, extend survival in a targeted, biomarker-driven way.
But it’s not without trade-offs. These drugs require careful management of toxicities, especially neutropenia and hyperglycaemia. Patients with diabetes were excluded, which is not reflective of the real world. A multidisciplinary approach is critical to support these patients.
Still, INAVO120 offers a hopeful path forward. For a population underserved by existing endocrine strategies, this may be the beginning of a new standard.”

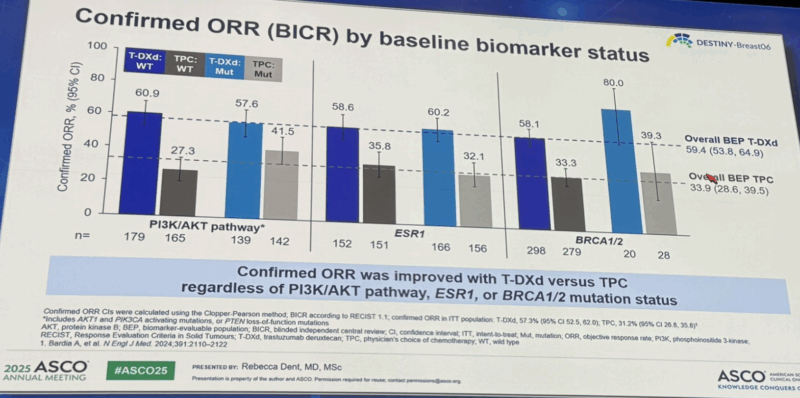
“Biomarker analyses from DB06 presented by Rebecca Dent.
As expected, the benefit of T-DXd over chemo is seen irrespective of tumor genomics (similar to DB04). Most dramatic benefit in the BRCAmut subset, though small numbers and in contrast with other trials (DB03/DB04/RWD)”
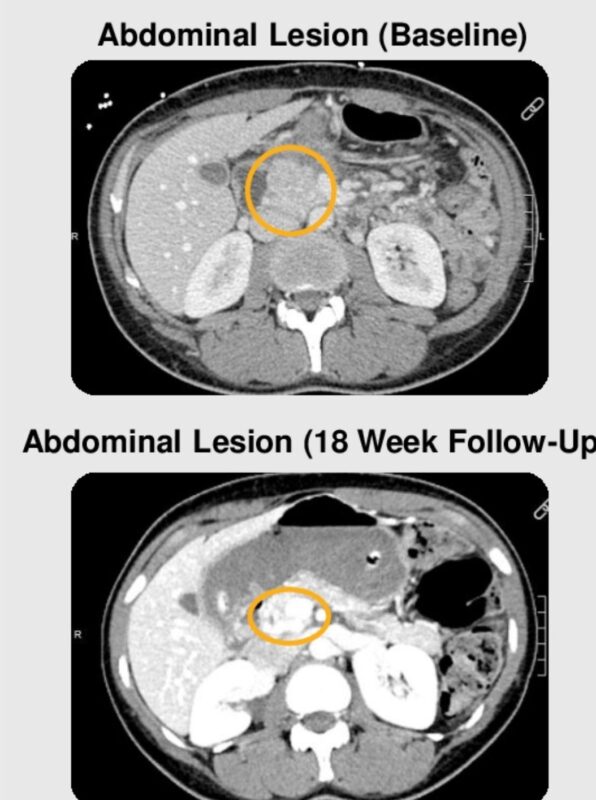
“Taking a bite out of SCLC with DLL3-directed therapies like tarlatamab, a first-in-class BiTE showing promise even in intracranial disease.
Precision immunotherapy is finally making inroads in this tough cancer type.”
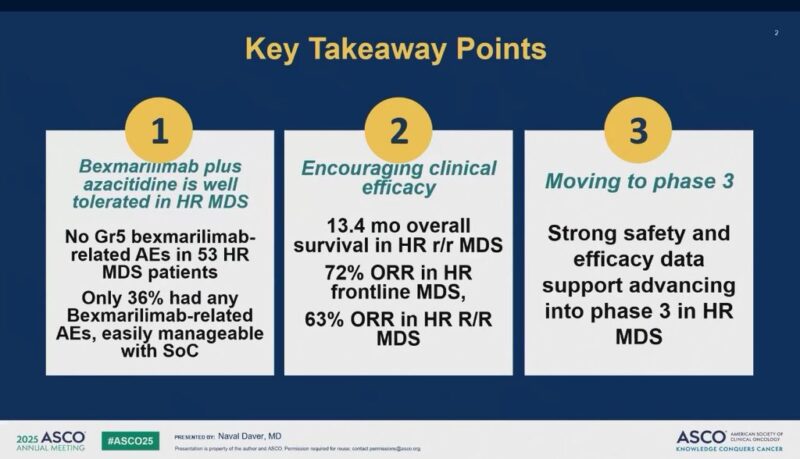
“Phase 1/2 study in HR-MDS presented by Dr. Naval Daver.
Bexmarlimab (CLEVER-1 macrophage immune checkpoint inhibitor) in combination with AZA.
- ORR 72% in frontline and 63% in RR (post HMA),
- mOS in RR MDS 13.4,
- Overall > 40% of patients had TP53m.”
“Interim analysis for 9MW2821 (Nectin4/MMAE ADC) and Toripalimab (n=40) RR =80%.
PFS=1 year. Small numbers and short follow up but it looks a bit like EVP, with slightly different toxicity. Randomised phase 3 announced in China.”
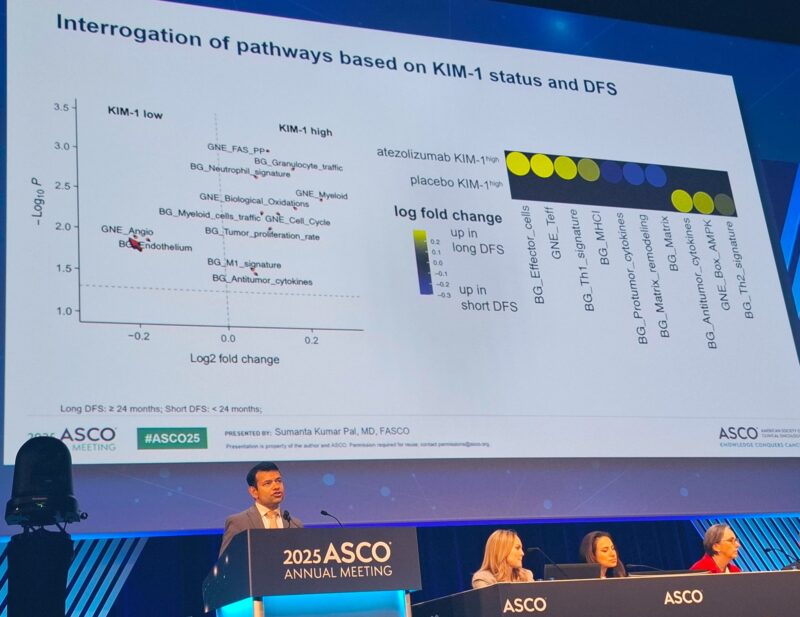
“Fascinating data from ASCO25!
Sumanta K. Pal presented genomic insights from IMmotion010. KIM-1 remains the most robust predictor of atezo benefit, with NMF6 (stromal/proliferative) tumors also showing promising DFS.
Important steps toward personalized adjuvant therapy in RCC.”
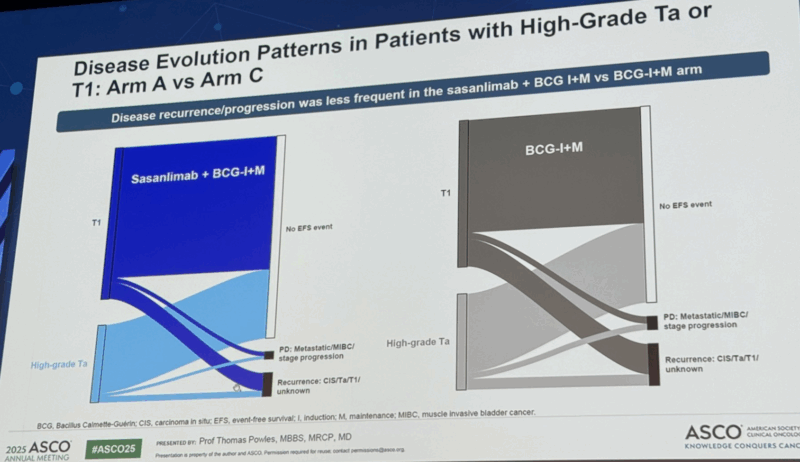
“CREST (sasanlimab + BCG ) shows consistent EFS benefit across stages.
Sankey plots show benefit driven mainly in NMIBC recurrence, but PS seems similar between arms. Is IO ready for prime time in BCG unexposed BC?”
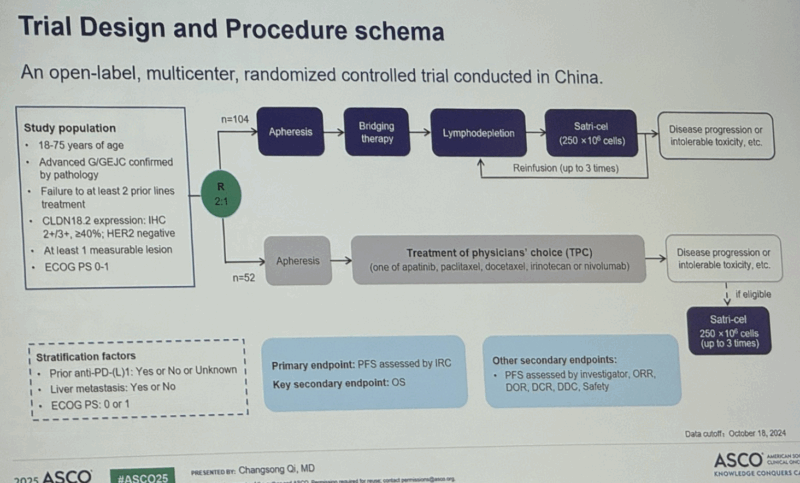
“First trial of CAR-T cell treatment for solid tumor response.
Satricabtagene autoleucel (satri-cel)/CT041 demonstrated significant progression-free survival (PFS) improvement and a clinically meaningful overall survival (OS) benefit in patients with previously treated, advanced G/GEJC.
Globally, this is the first ever randomized controlled trial of a CAR T-cell therapy in solid tumors to achieve superiority.
Satri-cel showed a manageable safety profile consistent with previous phase I results.
This trial expanded the percentage of CLDN18.2-positive patients with G/GEJC. These results support satri-cel as a new treatment option for advanced G/GEJC.”
“5 year results from KEYNOTE-564: Adjuvant pembrolizumab in ccRCC
- DFS not reached vs 68.3 mo → HR 0.71 (95% CI: 0.59–0.86),
- OS HR 0.66 (95% CI: 0.48–0.90), median OS not reached in both arms,
- 5-year DFS rate: 60.9% (pembro) vs 52.2% (placebo),
- 5-year OS rate: 87.7% vs 82.3%,
- Benefit confirmed across subgroups: intermediate-high, high risk, M1 NED, and sarcomatoid.
- No new serious treatment-related AEs in over 3 years.”

To stay on top of everything happening at ASCO 2025, keep following OncoDaily.
See also:
Written by Vahe Grigoryan


