Presented by Dr. Michiel S. Van Der Heijden (Netherlands Cancer Institute, Amsterdam) at the ASCO Annual Meeting 2025, the final results from the phase III CheckMate 901 trial (NCT03036098) provide new insight into first-line treatment strategies for cisplatin-ineligible patients with previously untreated, unresectable, or metastatic urothelial carcinoma (mUC).
What is the CheckMate 901 Trial?
CheckMate 901 is a global, randomized, open-label phase III trial evaluating the chemotherapy-free combination of nivolumab (NIVO) and ipilimumab (IPI) versus standard platinum-based chemotherapy (gemcitabine + carboplatin) in cisplatin-ineligible patients with mUC.
This subgroup of patients typically has poorer outcomes due to limited treatment options. The trial aimed to determine whether dual immune checkpoint blockade could improve overall survival (OS) and provide a more durable response.
CheckMate 901 Study Design and Patient Enrollment
A total of 445 patients with histologically confirmed, previously untreated unresectable or metastatic urothelial carcinoma who were ineligible for cisplatin were randomized 1:1 to receive:
- Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg every 3 weeks for up to 4 cycles, followed by nivolumab 480 mg every 4 weeks
- Gemcitabine + carboplatin every 3 weeks for up to 6 cycles
Stratification was based on PD-L1 expression and the presence of liver metastases.

Key Findings of CheckMate 901
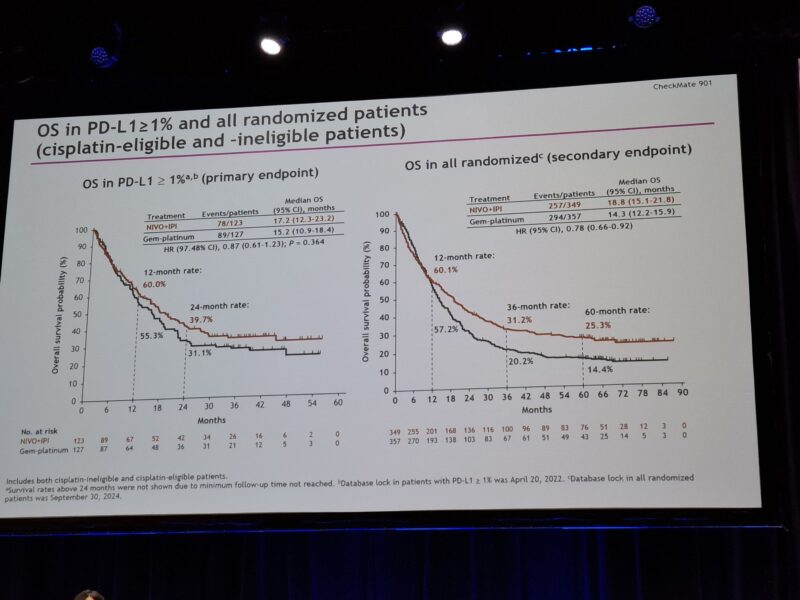
At ASCO 2025, updated results from the trial comparing nivolumab plus ipilimumab (NIVO+IPI) to standard gemcitabine and carboplatin (Gem-Carbo) in Cisplatin-ineligible patients with previously untreated unresectable or metastatic urothelial carcinoma (mUC). While the trial did not reach its prespecified threshold for statistical significance in overall survival (OS), the immunotherapy combination demonstrated clinically meaningful benefits.
The median overall survival was 19.1 months with NIVO+IPI compared to 13.2 months with Gem-Carbo, yielding a hazard ratio (HR) of 0.79 (98.27% CI, 0.61–1.01; P = 0.0245). Although this P-value did not meet the strict criteria for statistical significance, the survival advantage was notable. One-year OS rates were 59.7% for the immunotherapy group versus 54.3% for chemotherapy, and at three years, the gap widened further—29.6% versus 19.3%, respectively.
Progression-free survival (PFS) was similar between arms, with a median of 5.3 months for NIVO+IPI and 5.9 months for Gem-Carbo (HR: 0.90; 95% CI, 0.72–1.12). However, the long-term PFS rates strongly favored the immunotherapy regimen. At 12 months, PFS was 31.5% with NIVO+IPI versus 17.2% with chemotherapy. At 36 months, the rates were 20.0% and 4.9%, respectively.
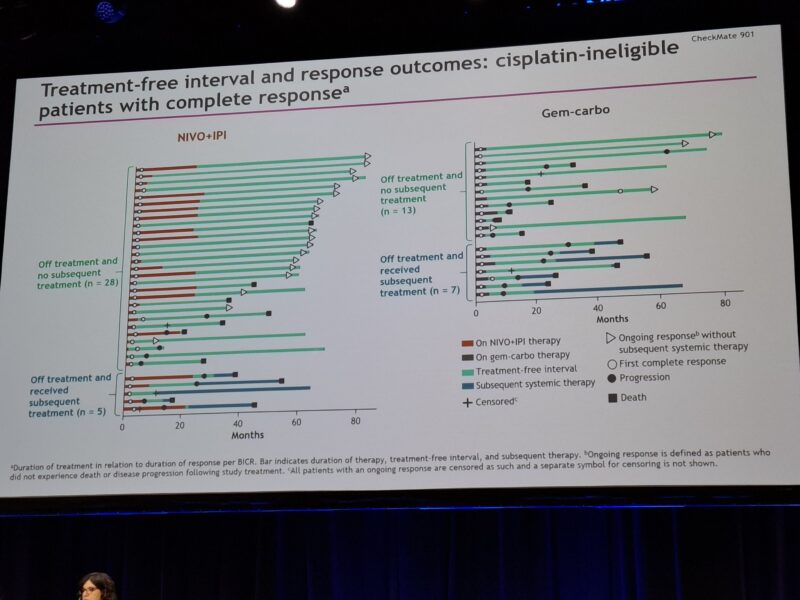
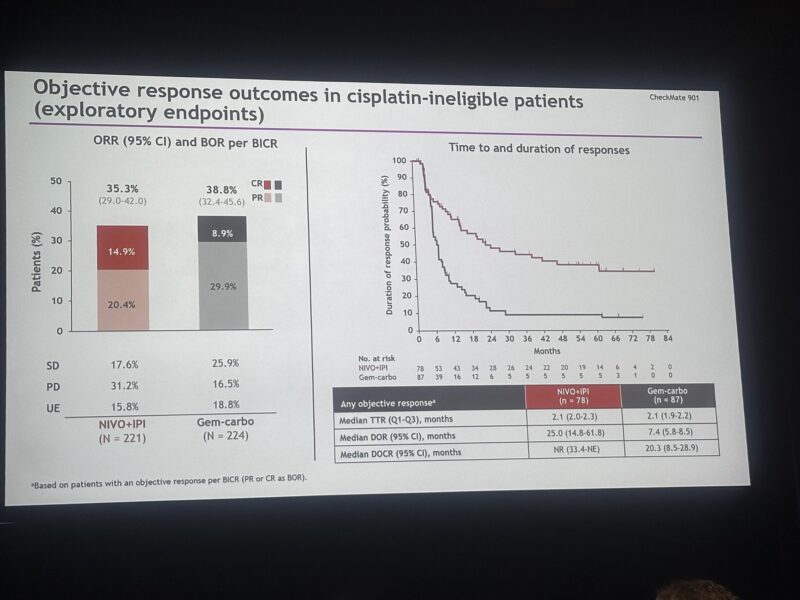
Importantly, the median duration of response (mDOR) was significantly longer in the NIVO+IPI arm—25.0 months compared to just 7.4 months with Gem-Carbo. These results highlight the potential of immunotherapy to deliver more durable responses and extended survival in patients with advanced biliary tract cancer, even in the absence of a statistically significant OS benefit under the trial’s strict criteria.
Safety Profile of Nivolumab Plus Ipilimumab vs Chemotherapy
The safety profile of nivolumab plus ipilimumab (NIVO+IPI) was generally manageable but distinct from that of standard chemotherapy in patients with advanced biliary tract cancer. Treatment-related adverse events (TRAEs) of any grade occurred in 89.0% of patients receiving NIVO+IPI, compared to 92.9% with gemcitabine plus carboplatin (Gem-Carbo). However, severe (grade 3–4) TRAEs were significantly less frequent with immunotherapy—47.2% versus 76.3% in the chemotherapy arm.
Despite the lower rate of severe toxicities, treatment discontinuation due to TRAEs was more common in the NIVO+IPI group (31.2%) compared to chemotherapy (14.2%), likely reflecting the immune-related nature of some adverse events. Treatment-related deaths occurred in seven patients in the NIVO+IPI arm and one patient in the Gem-Carbo arm.
No new safety signals were identified, though the immune-related adverse event profile of NIVO+IPI necessitates experienced management.
Read Full Abstract on ASCO 2025 Website
What People Are Saying About CheckMate 901?
Dr. Toni Choueiri shared updated findings from the CheckMate 901 trial on his X page, writing:
“ASCO25 | CheckMate 901 | Cis‑ineligible mUC: NIVO 1 mg/kg + IPI 3 mg/kg Q3W×4 → NIVO 480 mg Q4W vs gem‑carbo Q3W×6:
Any‑grade TRAEs 89% (G3–4 47%) vs 93% (G3–4 76%);
TRAE‑related discontinuation 31% vs 14%;
8 toxicity‑related deaths (7 vs 1);
no OS benefit in NIVO+IPI arm (not significant)”
Dr. Yüksel Ürün shared his involvement as a co-author on the CheckMate 901 trial via his X page, writing:
“Privilege to be one of the co-authors!
CheckMate 901: NIVO+IPI vs Gem-Carbo in cisplatin-ineligible mUC.
✔ OS at 5 years: 23.0% vs 14.4%
✔ mOS: 19.1 vs 13.2 months (HR: 0.79)
✔ Durable responses even off-treatment
But primary endpoint not met for PD-L1 ≥1%”
Clinical Implications and Takeaways
While the primary OS endpoint was not statistically met, CheckMate 901 marks the first phase III trial to demonstrate a chemotherapy-free, immune-based regimen offering durable responses and long-term survival benefit in cisplatin-ineligible mUC. The findings support the potential role of NIVO+IPI as an alternative to platinum-based chemotherapy, especially for patients seeking finite-duration treatment with sustained benefit. The results also reinforce the concept of long-tail survival in urothelial cancer with immunotherapy, previously seen in earlier phase studies.
More posts featuring ASCO25.