Opdivo (nivolumab) is a monoclonal antibody and immunotherapy drug which is a revolutionary approach that harnesses the body’s immune system to fight cancer. Unlike traditional treatments such as chemotherapy or radiation, which directly target cancer cells, immuno-oncology therapies work by enhancing the immune system’s ability to recognize and destroy tumors. Nivolumab functions as a programmed death receptor-1 (PD-1) blocking antibody, enhancing the body’s immune response against cancer cells. Opdivo has received approval from the U.S. Food and Drug Administration (FDA) for various cancer treatments.
How does Opdivo/Nivolumab work?
Nivolumab is an IgG4 monoclonal antibody that binds to PD-1 receptors with high affinity and specificity. T-cells express PD-1, which inhibits their immunological response when it binds to APC ligands PD-L1 and PD-L2. The regulation of both central and peripheral tolerance depends heavily on this system. Tumor cells can evade immune detection and destruction in cancer, nevertheless, because PD-1-expressing tumor-infiltrating lymphocytes are rendered inactive by abnormal PD-L1 expression by tumor cells or immune cells in the tumor microenvironment. Nivolumab frees immune cells from pathological immunological suppression by blocking PD-1 function, which enables them to identify and combat tumor cells.
Which company produced Opdivo?
Nivolumab is produced by Bristol Myers Squibb (BMS), a global biopharmaceutical company headquartered in New York, USA. BMS focuses on discovering, developing, and delivering innovative medicines in oncology, hematology, immunology, and cardiovascular diseases. The company has been a leader in cancer immunotherapy, with nivolumab (Opdivo) being one of its flagship drugs. Bristol Myers Squibb manufactures nivolumab in multiple dosages to accommodate different treatment regimens. The available vial strengths include:
- 40 mg/4 mL (10 mg/mL)
- 100 mg/10 mL (10 mg/mL)
- 240 mg/24 mL (10 mg/mL)
The patent for nivolumab is set to expire in 2028.
Which means that after this date, other pharmaceutical companies may be able to produce biosimilar versions of the drug. This could lead to increased competition and potentially lower costs for patients.
What Cancers is Opdivo Approved to Treat?
Opdivo is a key immunotherapy approved for several cancers, particularly for patients whose disease has progressed after standard treatments. In non-small cell lung cancer (NSCLC), it is used for metastatic cases following platinum-based chemotherapy. Similarly, in small cell lung cancer (SCLC), Opdivo provides a treatment option after prior chemotherapy.
For melanoma, Opdivo is approved for patients with unresectable or metastatic disease, either alone or in combination with Yervoy, improving survival outcomes. In renal cell carcinoma (RCC), it is used for both previously treated patients and those newly diagnosed with intermediate or poor risk, often in combination with ipilimumab.
In hepatocellular carcinoma (HCC), Opdivo is approved for patients who have already received sorafenib, either alone or with Yervoy, offering survival benefits. It is also used for recurrent or metastatic head and neck cancers following platinum-based therapy.
For esophageal squamous cell carcinoma, Opdivo is an option after prior chemotherapy with platinum and fluoropyrimidine. It is also approved for metastatic colorectal cancer with MSI-H or dMMR in patients 12 years and older, either alone or with ipilimumab.
You Can Read More About Immunotherapy for Colon Cancer on OncoDaily
In classical Hodgkin lymphoma, Opdivo is effective for relapsed cases after stem cell transplant and brentuximab vedotin or after multiple systemic therapies. Opdivo is approved for pleural mesothelioma in combination with ipilimumab for unresectable cases.
In advanced urothelial carcinoma, it is used after progression on platinum chemotherapy or within 12 months of neoadjuvant or adjuvant treatment. These approvals highlight the expanding role of Opdivo across multiple cancer types, offering new hope for patients with difficult-to-treat diseases.
Opdivo for Metastatic Cancer
In metastatic settings, Opdivo has shown promise in treating advanced cancers such as lung cancer, melanoma, and liver cancer. By targeting the PD-1 pathway, it helps control tumor progression and has been associated with improved survival rates. Case studies and pivotal clinical trials support its efficacy in these contexts.
Opdivo Combinations and Treatment Outcomes
Combining Opdivo with other treatments has been a strategy to enhance patient outcomes. In NSCLC, combining Opdivo with platinum-doublet chemotherapy has been explored as a neoadjuvant treatment, followed by Opdivo monotherapy after surgery. This approach aims to improve outcomes compared to chemotherapy alone.
The combination of Opdivo and Yervoy has been approved for certain patients with melanoma and has shown improved survival rates. This combination leverages the complementary mechanisms of both drugs to enhance the immune response against cancer cells.

The CheckMate 067 trial with a 7.5-year follow-up showed that nivolumab plus ipilimumab significantly improves long-term survival in advanced melanoma. The combination therapy achieved a median overall survival (OS) of 72.1 months (~6 years), with over 50% of patients surviving beyond 10 years. In contrast, pembrolizumab monotherapy had a median OS of 36.9 months (~3 years). Progression-free survival (PFS) was also longer with the combination (~11.5 months vs. 6.9 months for pembrolizumab). While nivolumab plus ipilimumab offers superior long-term outcomes, it comes with higher toxicity, making pembrolizumab a safer alternative for some patients.
The advantages of combining Opdivo with targeted therapy for a variety of malignancies have been highlighted by recent research. The CheckMate-9ER trial showed that the combination of nivolumab (Opdivo) and cabozantinib (Cabometyx) significantly improved survival in patients with advanced renal cell carcinoma (RCC) compared to sunitinib. Patients receiving the combination had a 30% lower risk of death, with median overall survival (OS) not reached, while those on sunitinib had a median OS of 29.5 months. The combination also nearly doubled progression-free survival (PFS), delaying disease progression for a median of 16.6 months versus 8.3 months with sunitinib. These results established nivolumab plus cabozantinib as a superior first-line treatment option for advanced RCC.
Read more about Immunotherapy for Bladder Cancer on OncoDaily
The CheckMate-9DW trial showed that nivolumab (Opdivo) plus ipilimumab (Yervoy) significantly improved overall survival (OS) in patients with advanced hepatocellular carcinoma (HCC). Patients on this combination had a median OS of 23.7 months, compared to 20.6 months with lenvatinib or sorafenib, reducing the risk of death by 21%. While progression-free survival (PFS) was similar between groups (9.1 vs. 9.2 months), long-term benefits were more pronounced, with 34% of patients progression-free at 18 months compared to 18% in the control group. These results reinforce nivolumab plus ipilimumab as a strong first-line option for advanced HCC.
Phase II ECOG3311 trial explores treatment de-intensification for high-risk, resected HPV-associated OPSCC by incorporating nivolumab into a reduced-dose radiation regimen. With promising 3-year overall survival (97%) and progression-free survival (86%), the approach appears effective while minimizing toxicity compared to standard therapy. The most common severe adverse event was lymphocytopenia, with a low rate of severe dysphagia or mucositis. Although one patient experienced fatal complications, the overall tolerability was favorable. Ongoing quality-of-life analyses will further clarify the benefits of this approach.
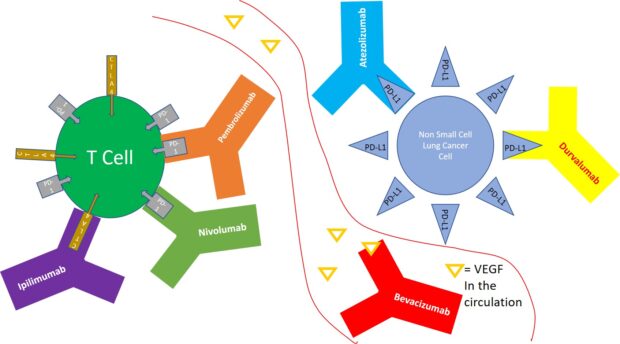
Opdivo is being tested in combination with anti-VEGF drugs like bevacizumab and ramucirumab in non-small cell lung cancer (NSCLC) to improve treatment outcomes. These results support the idea that immunotherapy and VEGF inhibitor combos can improve the prognosis of cancer.
The Side Effects of Opdivo and its Management
Many of Opdivo’s side effects are immune-related adverse effects, such as the immune system attacking normal organs and tissues which includes pneumonitis, colitis, hepatitis, and endocrinopathies.
- Pneumonitis is an inflammation of the lungs, presenting as a cough, shortness of breath, and chest pain.
- Colitis affects the colon, leading to diarrhea, abdominal pain, and even blood in the stool.
- Hepatitis, an inflammation of the liver, manifests with jaundice, dark urine, and elevated liver enzymes.
- Endocrinopathies result from immune-related dysfunction of hormone-producing glands, causing fatigue, weight changes, and mood disturbances.
Proactive management of side effects is essential to ensure patient safety and treatment success. Regular monitoring through blood tests and imaging allows early detection of complications before they become severe. Patients should report any new or worsening symptoms immediately to their healthcare team, as prompt intervention can prevent serious consequences. In cases of severe immune-related reactions, corticosteroids or other immunosuppressive drugs may be required to control the overactive immune response.
For lung cancer patients receiving Opdivo, pneumonitis is a particularly concerning side effect. Symptoms such as cough, fatigue, and shortness of breath can overlap with the underlying disease, making early detection crucial. Patients should report respiratory symptoms as soon as they arise, allowing for timely imaging and assessment. Treatment may involve temporarily discontinuing Opdivo or adjusting the dosage to minimize lung inflammation while still maintaining treatment efficacy.
Melanoma patients are more likely to experience skin-related immune responses, including rash, vitiligo (loss of skin pigment), and intense itching. Additionally, they may develop colitis or hepatitis due to immune activation. Managing these side effects requires a tailored approach. Mild skin reactions can often be controlled with topical treatments like moisturizers and corticosteroid creams, while more severe cases may necessitate systemic corticosteroids. Liver function monitoring through regular blood tests is also vital to detect early signs of hepatitis.
Gastric cancer patients receiving Opdivo often face gastrointestinal-related immune side effects. Nausea, appetite loss, and stomach inflammation can significantly impact their quality of life. To manage these symptoms, dietary adjustments such as small, frequent meals and nutritional supplementation can help maintain adequate nutrition. Medications like antiemetics can control nausea, while proton pump inhibitors can reduce stomach inflammation.
Hepatitis is one of the more severe immune-related side effects, leading to elevated liver enzymes and jaundice. Regular liver function tests are essential to detect any abnormalities early. Patients should also watch for symptoms like fatigue, abdominal pain, and yellowing of the skin or eyes. If liver inflammation is detected, treatment may involve temporary discontinuation of Opdivo and the administration of corticosteroids to suppress immune overactivation.

While most immune-related adverse effects can be managed with early intervention, some complications, such as cytokine release syndrome and organ-specific toxicities, can be life-threatening. Cytokine release syndrome causes systemic inflammation, leading to fever, fatigue, and dangerously low blood pressure. Severe cases of pneumonitis, colitis, or hepatitis can also become critical. In such instances, immediate medical attention is necessary. Opdivo may need to be discontinued, and high-dose corticosteroids or other immunosuppressants may be required to control the immune response.
Communication With Healthcare Providers is Essential. For patients undergoing Opdivo therapy, maintaining open communication with their healthcare team is crucial. Reporting symptoms early allows for rapid intervention and reduces the risk of complications. Regular monitoring and proactive management strategies significantly improve patient outcomes, ensuring the benefits of immunotherapy outweigh the risks.
What is the Recommended Dosage of Opdivo?
Nivolumab (Opdivo) is administered at varying dosages depending on the type of cancer being treated and whether it is used alone or in combination with other therapies. As a single agent, it is commonly given at a dose of 240 mg every two weeks or 480 mg every four weeks, delivered as a 30-minute intravenous infusion.
In unresectable cancers, it can be combined with other drug groups. NSCLC (tumor ≥4 cm or node-positive), is given at 360 mg every three weeks for three cycles with platinum-doublet chemotherapy. It is combined with ipilimumab at 360 mg every three weeks alongside 1 mg/kg of ipilimumab every six weeks, followed by nivolumab monotherapy for metastatic NSCLC.
For Stage IIB–IV melanoma, the combination with ipilimumab is given at 1 mg/kg alongside 3 mg/kg of ipilimumab every three weeks for four doses, followed by maintenance nivolumab at 240 mg every two weeks or 480 mg every four weeks. In metastatic gastroesophageal junction cancer, it is used at 240 mg every two weeks or 480 mg every four weeks with fluoropyrimidine- and platinum-based chemotherapy, continuing until disease progression or intolerable toxicity.
What to Avoid During Opdivo Treatment?
Undergoing Opdivo (nivolumab) therapy involves several stages, each with important considerations. Before starting treatment, the healthcare team will perform blood tests and imaging scans to assess the patient’s overall health and establish a baseline. It is essential to inform the medical team about any existing conditions, allergies, or medications to ensure the safest and most effective treatment plan.
Opdivo is administered intravenously, typically over 30 to 60 minutes. During the infusion, the patient will be closely monitored for any immediate reactions. To stay comfortable, bringing a caregiver, a book, music, or anything that helps with relaxation can be beneficial. After the infusion, regular follow-ups, including blood tests and scans, will help track progress. Common side effects such as fatigue, skin reactions, or digestive issues can often be managed with medications and lifestyle adjustments. Emotional support is also crucial, and connecting with counselors, support groups, or loved ones can make a significant difference.
Certain medications, supplements, and lifestyle choices should be approached with caution. Immunosuppressants, including corticosteroids, may reduce Opdivo’s effectiveness, so it is important to discuss all medications with the healthcare provider. While no specific foods interfere with treatment, maintaining a well-balanced diet supports overall health. Moderate alcohol consumption may be permissible, but it should be discussed with a doctor. Smoking can impair immune function and is strongly discouraged during cancer treatment.
By staying informed, following medical advice, and maintaining open communication with the healthcare team, the patient can navigate Opdivo therapy with confidence and optimize treatment outcomes.
Opdivo’s Effectiveness Over Time
Opdivo (nivolumab) has shown remarkable long-term efficacy across multiple cancers, with clinical trials demonstrating significant improvements in overall survival and progression-free survival. In advanced melanoma, a landmark study reported that more than half of the patients treated with a combination of nivolumab and ipilimumab survived for at least ten years, highlighting the potential for durable responses.
Similarly, in advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma, the CheckMate 649 trial confirmed that nivolumab combined with chemotherapy provided meaningful long-term survival benefits compared to chemotherapy alone, with sustained improvements seen at five years of follow-up. For patients with advanced renal cell carcinoma, updated analyses from the CheckMate 214 trial, with a median follow-up of eight years, demonstrated superior survival benefits with nivolumab plus ipilimumab compared to sunitinib. In head and neck squamous cell carcinoma, a retrospective study indicated a median progression-free survival of three months, with the survival curve stabilizing after three years, suggesting a potential long-term benefit for a subset of patients.
The duration of Opdivo treatment varies depending on cancer type, disease stage, and individual response. In non-small cell lung cancer, therapy is typically continued until disease progression or the development of unacceptable toxicity. For advanced melanoma, treatment may extend up to two years, particularly in cases where patients respond well. While long-term therapy is generally well-tolerated, there remains a risk of immune-related adverse events, making regular monitoring essential to assess treatment effectiveness and manage side effects.
The cost of Opdivo can be substantial, with expenses varying based on dosage, duration, and geographic location. In the United States, treatment can cost several thousand dollars per infusion. To support patients, Bristol-Myers Squibb offers financial assistance programs such as Access Support, which guides insurance coverage and potential financial aid. Patients concerned about affordability are encouraged to discuss options with their healthcare providers and explore available assistance programs. Currently, biosimilars to Opdivo are not widely available, and any future alternatives should be carefully evaluated based on emerging clinical data and regulatory approvals.
Is Opdivo Immunotherapy or Chemotherapy?
Opdivo is classified as an immunotherapy drug. While chemotherapy directly targets rapidly dividing cancer cells, Opdivo works by inhibiting the PD-1 pathway, thereby enhancing the immune system’s ability to recognize and attack cancer cells. This approach represents a significant advancement in oncology, offering new avenues for treatment with potentially different side effect profiles compared to conventional chemotherapy. In summary, Opdivo has played a pivotal role in advancing cancer treatment through its immunotherapeutic approach.

You can read more about main differences of Immunotherapy vs Targeted Therapy on OncoDaily
Facts about Nivolumab
In December 2024, the FDA approved a subcutaneous formulation of nivolumab for patients with advanced or metastatic solid tumors. This approval was based on clinical trials demonstrating comparable efficacy and safety profiles between subcutaneous and intravenous administrations. The subcutaneous route offers a more convenient option for patients, potentially improving treatment adherence.
A phase 3 randomized study evaluated the addition of low-dose nivolumab to metronomic chemotherapy (MC) in advanced head and neck squamous cell carcinoma (HNSCC), aiming to improve survival in resource-limited settings. The trial included 151 patients, comparing MC alone to MC plus low-dose nivolumab (MC-I). The addition of nivolumab significantly improved 1-year overall survival (OS) from 16.3% to 43.4% (HR 0.545, P=0.00358), with a median OS increase from 6.7 to 10.1 months (P=0.0052). Progression-free survival (PFS) also improved from 4.57 to 6.57 months (P=0.0021), while response rates increased from 49.3% to 65.2% (P=0.085). Adverse events were similar between groups. This study suggests that low-dose nivolumab is a viable alternative for patients unable to access standard immunotherapy.
A single-center, phase 2 open-label clinical trial (NCT05772624) was conducted to evaluate the safety and efficacy of low-dose nivolumab in combination with AVD as a first-line treatment for classical Hodgkin lymphoma. The study aimed to make the treatment more accessible, especially in low- to middle-income countries, by reducing the cost associated with standard doses. A large NCI-funded clinical trial updated its results in late 2024, indicating that nivolumab combined with a three-drug chemotherapy regimen (AVD) is more effective than the current standard treatment for advanced-stage classic Hodgkin lymphoma. This combination improved cancer elimination and progression-free survival rates.
A randomized Phase II trial (NCT06325683) is investigating the combination of nivolumab and relatlimab versus standard care (lomustine) for patients with recurrent glioblastoma. The study aims to assess the efficacy of this combination in improving patient outcomes.
A clinical trial led by the Peter MacCallum Cancer Centre demonstrated significant improvements in breast cancer cure rates by adding nivolumab to pre-surgery chemotherapy. The trial involved over 500 women with early-stage, high-risk breast cancer, and the addition of nivolumab nearly doubled the rate of pathological complete response.
You Can Read More on Official Website
Written by Mariam Khachatryan, MD
FAQ
Can Opdivo be used for cancers without PD-L1 expression?
Yes, Opdivo has shown efficacy in certain cancers regardless of PD-L1 expression levels. For example, in the CheckMate -816 trial, neoadjuvant Opdivo combined with chemotherapy provided benefits for patients with resectable non-small cell lung cancer across all PD-L1 expression levels.
What should I do if I experience severe side effects during Opdivo treatment?
If you experience severe side effects such as pneumonitis, colitis, or hepatitis during Opdivo treatment, contact your healthcare provider immediately. Early recognition and prompt management are crucial. Keep a detailed record of your symptoms, including their onset and severity, to assist your medical team in providing appropriate care.
Is Opdivo safe for long-term use?
Long-term use of Opdivo is generally considered safe for certain cancers, but it may increase the risk of immune-related side effects. Regular monitoring by your healthcare provider is essential to manage potential adverse effects and assess ongoing treatment efficacy.
Can Opdivo be used alongside complementary therapies?
Combining Opdivo with complementary therapies, such as acupuncture or dietary changes, may help manage side effects or improve quality of life. However, it's important to consult your healthcare provider before starting complementary therapies to ensure they do not interfere with Opdivo's effectiveness or cause adverse interactions.
How long should Opdivo administration last?
Opdivo (Nivolumab) is usually adminsitred within 30-60 min in an IV line.
Can Opdivo be combined with chemotherapy?
Yes, Opdivo (Nivolumab) can be used with or without chemotherapy depending on specific indication.