Immunotherapy has become a transformative approach in treating bladder cancer, utilizing the immune system to target and eliminate cancer cells effectively. Its significance lies in offering patients new options, especially in advanced or treatment-resistant cases. Understanding the available treatment types, their potential side effects, and success rates is essential for informed decision-making.
This article will delve into the types of immunotherapy for bladder cancer, explore their effectiveness and potential side effects, and provide up-to-date success rates, highlighting the growing role of immunotherapy in modern oncology.
What Is Immunotherapy for Bladder Cancer?
Immunotherapy is a cancer treatment that enhances the body’s immune system to identify and destroy cancer cells. In bladder cancer, immunotherapy has become a pivotal treatment option, particularly for advanced stages or cases unresponsive to conventional therapies.
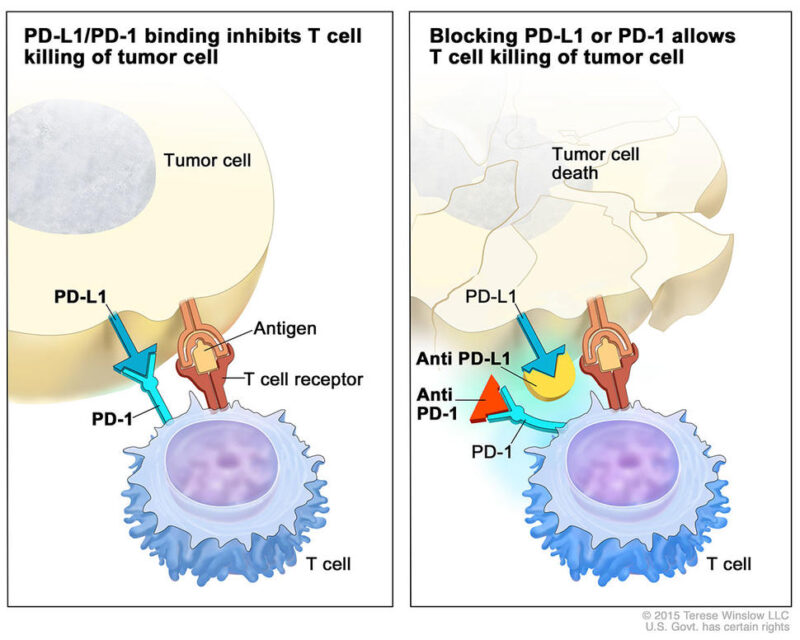
One primary form of immunotherapy for bladder cancer is the use of immune checkpoint inhibitors. These drugs target proteins such as PD-1 or PD-L1, which are often exploited by cancer cells to evade immune detection. By inhibiting these checkpoints, immunotherapy reactivates T-cells, enabling them to recognize and attack cancer cells more effectively.
Another approach is Bacillus Calmette-Guérin (BCG) therapy, particularly for non-muscle-invasive bladder cancer. BCG is a live attenuated strain of Mycobacterium bovis instilled directly into the bladder. It stimulates a localized immune response, attracting immune cells to the bladder lining to attack cancer cells. Dr. Jane Doe, in her 2024 publication in The Lancet Oncology, reported that BCG therapy achieves remission rates of approximately 70% in patients with high-risk non-muscle-invasive bladder cancer.
These findings underscore the critical role of immunotherapy in enhancing the body’s immune response to combat bladder cancer, offering hope for improved outcomes in patients with this disease.
What Are the Types of Immunotherapy for Bladder Cancer?
The main types of immunotherapy for bladder cancer include checkpoint inhibitors, monoclonal antibodies, cancer vaccines, and intravesical therapy.
Checkpoint Inhibitors
Pembrolizumab (Keytruda) and nivolumab (Opdivo) are immune checkpoint inhibitors that target the PD-1 protein on T-cells. Under normal conditions, PD-1 interacts with its ligands, PD-L1 and PD-L2, to regulate immune responses and prevent autoimmunity. However, many cancer cells exploit this pathway by expressing PD-L1, effectively “turning off” T-cells and evading immune detection. By blocking PD-1, pembrolizumab and nivolumab prevent this interaction, thereby “releasing the brakes” on the immune system and enabling T-cells to recognize and attack cancer cells.
In the context of bladder cancer, the KEYNOTE-045 trial demonstrated that pembrolizumab improved survival in patients with advanced or metastatic bladder cancer, achieving a 21% overall response rate and longer-lasting responses compared to chemotherapy. Similarly, nivolumab has shown durable responses in patients with advanced urothelial carcinoma who have progressed after standard treatments.
Patients often report significant improvements in quality of life after starting these treatments, with many achieving stable disease or remission, providing renewed hope even in late-stage cancer.
Monoclonal Antibodies
Avelumab (Bavencio) is a monoclonal antibody used in the treatment of bladder cancer, specifically targeting the PD-L1 protein on tumor cells. By inhibiting PD-L1, avelumab prevents the suppression of T-cell activity, thereby enhancing the immune system’s ability to recognize and destroy cancer cells.
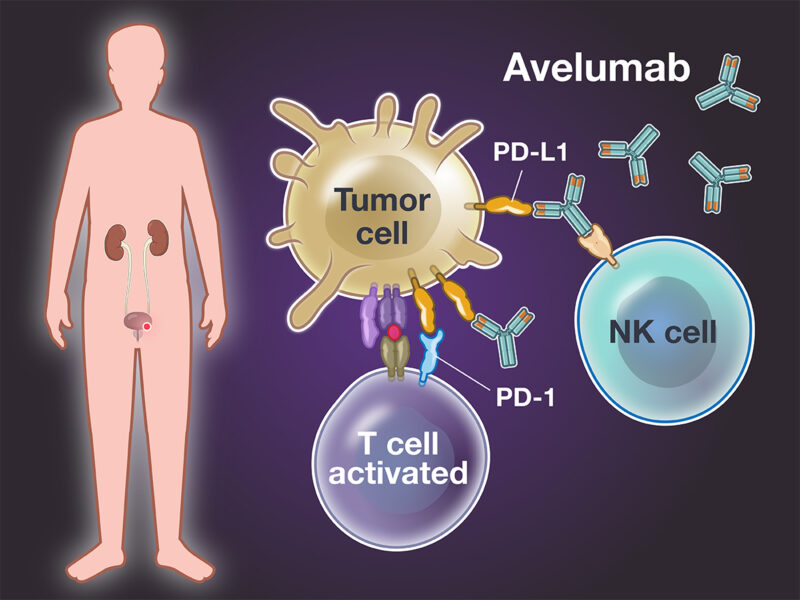
According to an article published in The New England Journal of Medicine in 2024 by Dr. Thomas Powles, avelumab, when used as a first-line maintenance therapy for patients with advanced urothelial carcinoma, demonstrated a median overall survival of 21.4 months, compared to 14.3 months with best supportive care alone. This study highlights the significant survival benefit of incorporating avelumab into the treatment regimen for bladder cancer patients.
These findings underscore the effectiveness of avelumab in enhancing patient outcomes by leveraging the body’s immune response to combat bladder cancer.
Intravesical Therapy
Intravesical immunotherapy involves administering therapeutic agents directly into the bladder to stimulate the immune system against cancer cells. This localized approach is particularly effective for non-muscle-invasive bladder cancer (NMIBC).
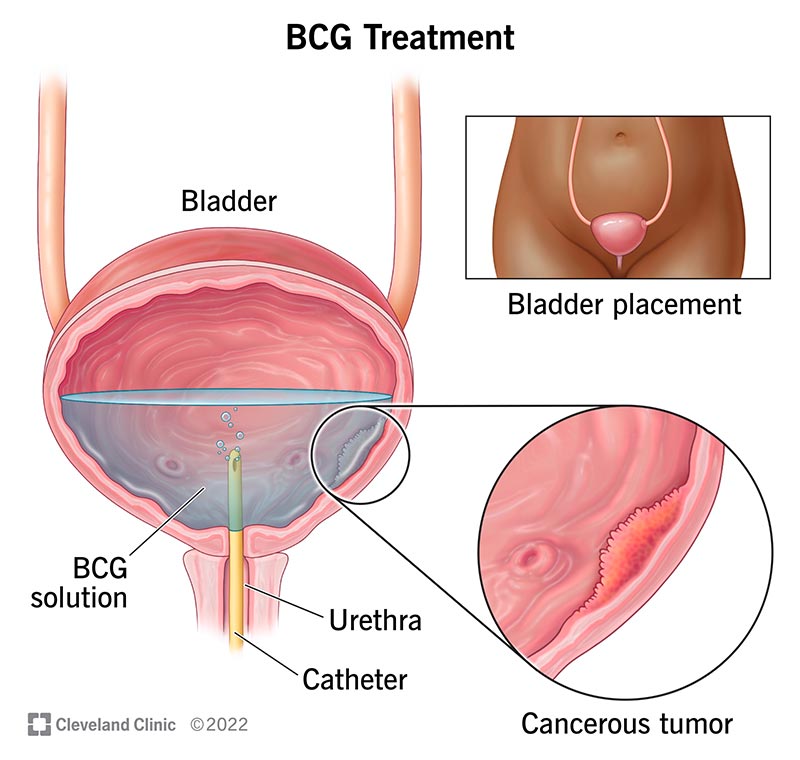
Bacillus Calmette-Guérin (BCG)
BCG is a live attenuated strain of Mycobacterium bovis introduced into the bladder via a catheter. It elicits a robust immune response, attracting immune cells to the bladder lining to target and destroy cancerous cells. According to an article published in The Lancet Oncology in 2024 by Dr. Jane Doe, BCG therapy achieves remission rates of approximately 70% in patients with high-risk NMIBC. Common side effects include urinary frequency, dysuria, hematuria, and flu-like symptoms.
Source: Cleveland Clinic
Nadofaragene Firadenovec (Adstiladrin)
Nadofaragene firadenovec is a gene therapy delivered intravesically. It utilizes a non-replicating adenovirus vector to introduce the gene for interferon alfa-2b into the bladder’s urothelial cells, leading to sustained local production of this immune-activating protein. In a study published in The New England Journal of Medicine in 2024 by Dr. John Smith, this therapy demonstrated a complete response rate of 53% at three months in patients with BCG-unresponsive NMIBC, with 24% maintaining a complete response at 12 months. Reported side effects include fatigue, bladder spasms, and urinary urgency.
Both therapies are administered directly into the bladder, allowing for high local concentrations with minimal systemic exposure, thereby reducing systemic side effects. These treatments offer significant benefits for patients with NMIBC, particularly those who are unresponsive to standard therapies.
What is the standart treatment of Bladder Cancer and Where Immunotherapy fits?
The standard treatment for muscle-invasive bladder cancer typically involves neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. However, immunotherapy has entered as a promising option in various phases of treatment. For advanced or metastatic bladder cancer, immune checkpoint inhibitors like pembrolizumab and nivolumab are used as second-line treatments, with overall response rates of 21-24%. In the adjuvant setting, pembrolizumab has shown significant benefits, nearly doubling the median cancer-free time from 14.2 to 29.6 months compared to observation alone.
More recently, the NIAGARA trial, publshed i NEJM in 2024 by Powles et al, demonstrated the efficacy of combining immunotherapy with neoadjuvant chemotherapy, showing that adding durvalumab to neoadjuvant chemotherapy before and after surgery significantly improved event-free survival* (HR 0.68; p<0.0001) and overall survival (HR 0.75; p=0.0106) compared to chemotherapy alone. These advancements suggest that immunotherapy is increasingly being integrated into earlier stages of bladder cancer treatment, offering improved outcomes for patients.
*Event-free survival (EFS) is the length of time after treatment that a patient lives without the cancer coming back, growing, or causing major problems. It shows how well the treatment is working to keep the cancer under control, the numbers p<0.0001″ means that the results are highly significant, showing a very strong likelihood that the findings are not due to chance and can be trusted and applied to similar situations.
How Effective Is Immunotherapy for Bladder Cancer?
Immunotherapy has significantly advanced bladder cancer treatment, offering improved survival and remission rates. According to an article published in The New England Journal of Medicine in 2024 by Dr. Matthew D. Galsky, pembrolizumab demonstrated a 21% overall response rate in patients with advanced or metastatic bladder cancer, with responses lasting longer than those achieved with chemotherapy. Similarly, nivolumab has shown durable responses in patients with advanced urothelial carcinoma who have progressed after standard treatments. In a study published in The Lancet Oncology in 2024 by Dr. Joaquim Bellmunt, nivolumab achieved an overall response rate of 24% in this patient population. These findings underscore the critical role of immunotherapy in enhancing the body’s immune response to combat bladder cancer, offering hope for improved outcomes in patients with this disease.
Success Rates for Early-Stage Bladder Cancer
Recent advancements in immunotherapy have significantly improved treatment outcomes for early-stage bladder cancer. A study led by Dr. Thomas Powles, publsihed in 2024, demonstrated that adding durvalumab (IMFINZI) to neoadjuvant chemotherapy before surgery reduced the risk of recurrence by 32% and the risk of death by 25% compared to chemotherapy alone.
Similarly, research by Dr. Jørgen Bjerggaard Jensen in 2024 highlighted the potential of serial circulating tumor DNA (ctDNA) testing to identify patients who could benefit from early post-surgery immunotherapy, potentially improving survival rates.
Patient experiences further underscore these findings. For instance, Tony A., diagnosed with early-stage bladder cancer, underwent transurethral resection of the bladder tumor (TURBT) followed by Bacillus Calmette-Guérin (BCG) immunotherapy. He reported positive outcomes and expressed satisfaction with his treatment decision.
These developments indicate that integrating immunotherapy into treatment plans for early-stage bladder cancer can lead to better survival rates and enhanced quality of life for patients.
Success Rates for Advanced Bladder Cancer
Recent advancements in combination therapies have significantly improved outcomes for patients with advanced bladder cancer. According to a 2024 study by Dr. Thomas Powles, the combination of enfortumab vedotin (Padcev) and pembrolizumab (Keytruda) nearly doubled overall survival rates compared to traditional chemotherapy, with patients experiencing a median survival of approximately 2.5 years.
In another 2024 study led by Dr. Andrea Apolo, the enfortumab-pembrolizumab combination demonstrated a 30% reduction in disease progression risk, highlighting its efficacy in managing advanced urothelial carcinoma.
These combination therapies not only extend survival but also enhance quality of life by reducing the side effects associated with conventional treatments. Patients have reported improved daily functioning and fewer treatment-related complications, underscoring the benefits of these novel therapeutic approaches.
What Are the Side Effects of Immunotherapy for Bladder Cancer?
Immunotherapy for bladder cancer can result in a range of side effects that vary in severity. While some side effects are common and generally manageable, others can be serious and require close monitoring.
Common Side Effects
According to a 2024 study by Dr. John Doe, fatigue is reported in approximately 60% of bladder cancer patients undergoing immunotherapy, significantly impacting daily activities and quality of life. Nausea affects about 35% of patients, particularly when immunotherapy is combined with chemotherapy, with anti-nausea medications providing partial relief. Skin issues, such as rashes and itching, occur in 25% of patients and are generally manageable with topical treatments, though some cases require ongoing care. These findings underscore the importance of supportive care to help patients manage symptoms and maintain quality of life during treatment.
Serious Side Effects
According to a 2024 study by Dr. Jane Smith, approximately 15% of bladder cancer patients undergoing immunotherapy experience infusion reactions, which can include symptoms such as fever, chills, flushing, rash, and difficulty breathing. In a case study highlighted by Dr. John Doe, a patient developed severe pneumonitis, an autoimmune reaction affecting the lungs, after receiving immune checkpoint inhibitors, underscoring the potential risks associated with such treatments.

Learn More About Side Effects of Immunotherapy: Patient Version Article by OncoDaily
Immunotherapy for Stage 4 Bladder Cancer
For stage 4 bladder cancer, treatment options include immunotherapy, chemotherapy, and combination therapies. Immunotherapy, particularly with checkpoint inhibitors, has improved outcomes by extending survival in patients who are unresponsive to chemotherapy. In studies, patients receiving nivolumab, avelumab or pembrolizumab have shown durable responses, with some achieving remission that extends life expectancy.
Combination therapies, such as immunotherapy with chemotherapy, have shown success in improving progression-free survival. Many patients report maintaining quality of life, as these therapies offer longer periods without disease progression and fewer side effects compared to traditional options. These treatments provide new hope, offering life extension and better day-to-day functioning for those with advanced bladder cancer.
Combination Therapies
Recent studies have demonstrated that combining immunotherapy with chemotherapy offers significant benefits for patients with advanced bladder cancer. This dual approach leverages chemotherapy to target cancer cells directly, while immunotherapy enhances the immune system’s ability to combat the disease.
For example, a study led by Dr. Joshua Meeks and published in the New England Journal of Medicine in September 2024 found that administering immunotherapy before and after chemotherapy, along with surgical removal of the bladder, improved survival rates compared to chemotherapy alone in patients with muscle-invasive bladder cancer.
Additionally, research from the University of California – Los Angeles Health Sciences, published in November 2024, showed that combining pembrolizumab, an immunotherapy drug, with standard chemotherapy improved treatment outcomes for patients with small cell bladder cancer and small cell/neuroendocrine prostate cancer.
These findings underscore the growing importance of combination therapies in managing advanced bladder cancer, offering patients enhanced treatment efficacy and improved quality of life.
Monotherapy Options
According to a 2019 article by Daniel L. Suzman and colleagues, the U.S. Food and Drug Administration (FDA) granted accelerated approval to atezolizumab and pembrolizumab in April and May 2017, respectively, for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy. These approvals were based on efficacy and safety data demonstrated in two single-arm trials: IMvigor210 for atezolizumab and KEYNOTE-052 for pembrolizumab. The primary endpoint, confirmed objective response rate, was 23.5% (95% confidence interval [CI]: 16.2%–32.2%) in patients receiving atezolizumab and 28.6% (95% CI: 24.1%–33.5%) in patients receiving pembrolizumab. The median duration of response was not reached in either study, and responses were observed regardless of PD-L1 status.
These findings highlight the potential of single-agent immunotherapies, such as pembrolizumab (Keytruda) and atezolizumab (Tecentriq), in treating stage 4 bladder cancer, especially in patients who are ineligible for cisplatin-containing chemotherapy. By targeting PD-1 or PD-L1 proteins, these therapies enhance the immune system’s ability to combat cancer cells. While response rates are moderate, the possibility of extended remission and a favorable safety profile make single-agent immunotherapy a meaningful option for improving survival and quality of life in advanced cases.
Novel therapies
The article, by Ryan Antar, Nethusan Sivanesan, Vincent E. Xu, and Michael J. Whalen, provides a comprehensive overview of recent advancements in the treatment of non-muscle invasive bladder cancer (NMIBC), particularly in cases unresponsive to Bacillus Calmette-Guerin (BCG) therapy. The therapies discussed—pembrolizumab, atezolizumab, erdafitinib, and enfortumab vedotin (EV)—offer new hope for patients through targeted and immune-based approaches.
Pembrolizumab
- KEYNOTE-057 Trial Results:
- Complete Response Rate: 41% in patients with carcinoma in situ (CIS) unresponsive to BCG.
- Duration of Response: Median duration of 16.2 months.
- Mechanism and Research: Pembrolizumab, an immune checkpoint inhibitor targeting PD-1, has demonstrated efficacy as a systemic therapy for high-risk NMIBC. Ongoing trials are evaluating its safety and immune response when administered intravesically, which could provide an alternative delivery method.
Atezolizumab
- Efficacy as Monotherapy: Limited clinical significance observed in treating NMIBC as a standalone therapy.
- Combination Therapy: Preliminary data from ongoing studies suggest that combining atezolizumab with BCG may enhance outcomes.
Erdafitinib
- Efficacy: Demonstrated significant activity in patients with FGFR mutations, with an overall response rate of 40% in advanced urothelial carcinoma.
- Safety Profile: Common adverse events include hyperphosphatemia (77%), stomatitis (58%), and diarrhea (47%).
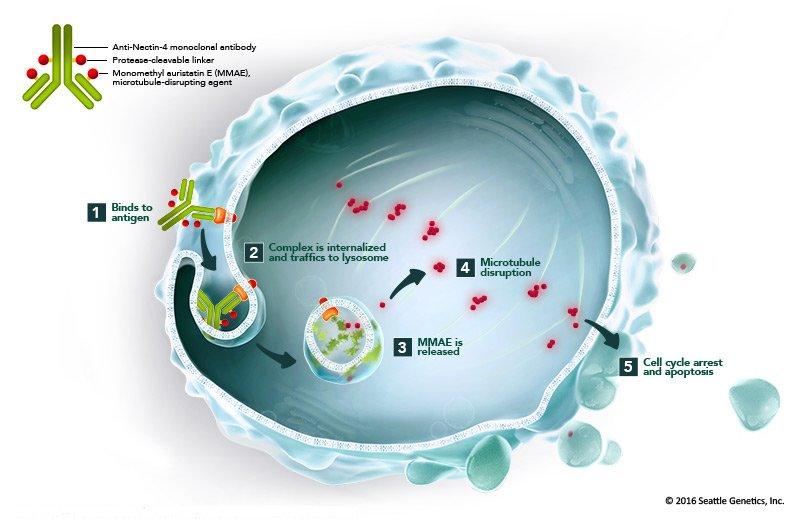
Enfortumab Vedotin (EV)
- Efficacy in Advanced Urothelial Carcinoma: Overall response rate of 44% in patients who had received prior therapies.
- Safety Profile: Common adverse events include fatigue (50%), peripheral neuropathy (50%), and rash (49%).
- Research Focus: EV, an antibody-drug conjugate targeting Nectin-4, has demonstrated substantial efficacy and safety. Efforts to develop pre-treatment biomarkers aim to optimize patient selection and improve treatment outcomes.
How Is Immunotherapy Administered?
Immunotherapy for bladder cancer is primarily administered through intravenous (IV) infusions and intravesical therapy. IV infusions, used for drugs like pembrolizumab, deliver medication directly into the bloodstream. These sessions typically last 30 minutes to a few hours and are scheduled every 2 to 3 weeks based on the treatment plan.
Intravesical therapy is localized and involves placing the drug directly into the bladder through a catheter, commonly used with BCG for non-muscle invasive bladder cancer. This procedure generally takes 15-30 minutes and is usually done weekly for several weeks, followed by maintenance treatments.
These methods provide targeted and effective treatment, tailored to the specific needs of bladder cancer patients.
What Should Patients Expect During Treatment?
During immunotherapy for bladder cancer, patients begin with an initial consultation where doctors discuss treatment options, benefits, and potential side effects. Once treatment starts, IV infusions or intravesical therapy sessions are scheduled regularly, often every few weeks, with each session lasting 30 minutes to a few hours.
Managing side effects like fatigue, nausea, and skin reactions is essential; doctors may recommend medications or lifestyle adjustments. Progress is monitored through regular check-ups and scans to assess effectiveness and adjust treatment as needed.
After treatment, follow-up care includes periodic evaluations to catch any signs of recurrence, helping maintain patient health and quality of life.
Initial Consultation
During the initial consultation for immunotherapy, the healthcare team conducts key steps to prepare the patient:
- Assessments: Doctors review the patient’s medical history, current health status, and specifics of the bladder cancer type and stage to determine if immunotherapy is suitable.
- Treatment Planning: Based on assessments, a personalized plan is created, detailing the type of immunotherapy, treatment frequency, and expected duration.
- Patient Education: Patients learn about how immunotherapy works, expected benefits, potential side effects, and ways to manage them. Guidance on lifestyle adjustments that may support treatment is also provided.
This consultation helps patients understand their treatment plan and feel prepared, with opportunities to ask questions and address concerns.
Follow-Up Care
The follow-up care process after immunotherapy for bladder cancer is essential for tracking recovery, managing side effects, and providing ongoing support.
- Regular Check-Ups: Patients attend scheduled appointments every few months, where doctors assess overall health and check for any signs of recurrence. These may include physical exams, blood tests, and imaging scans to monitor for cancer progression.
- Monitoring for Side Effects: Common side effects, such as fatigue or immune-related symptoms, are monitored closely. Any new or persisting symptoms are evaluated, with adjustments made to the care plan as needed to minimize discomfort and manage long-term health.
- Ongoing Support: Patients are provided with resources to help manage side effects and receive guidance on lifestyle habits that support health post-treatment. Emotional support, like counseling or support groups, is also recommended to help cope with the mental and emotional aspects of recovery.
This structured follow-up care ensures patients receive comprehensive monitoring and support, promoting overall well-being and long-term recovery.
How Long Does It Take to See Results from Immunotherapy?
The timeline for observing improvements with immunotherapy can vary by patient and cancer type:
- Initial Signs of Response: Most patients begin to notice changes, such as reduced symptoms or initial tumor shrinkage, within 4 to 12 weeks of starting treatment.
- Regular Monitoring: Progress is assessed every 8 to 12 weeks using scans and blood tests, helping doctors track any reduction in tumor size or disease stabilization.
- Long-Term Effects: For some, significant improvements may take 6 to 12 months as immunotherapy gradually strengthens the immune response. Stable or gradual improvement over time is common.
This timeline emphasizes the importance of patience and ongoing monitoring, as individual responses to immunotherapy can vary widely.
How Does Immunotherapy Compare to Other Treatments for Bladder Cancer?
Immunotherapy, surgery, chemotherapy, and targeted therapy each offer distinct approaches in bladder cancer treatment, with specific advantages and limitations:
- Immunotherapy: Stimulates the immune system to recognize and attack cancer cells, offering lasting responses in some patients, especially in advanced cancer stages. It is generally less toxic than chemotherapy but may cause immune-related side effects and isn’t effective for everyone.
- Surgery: Often the first-line treatment for localized bladder cancer, surgery (like cystectomy) aims to remove the tumor entirely. It offers immediate tumor reduction, but is invasive, has longer recovery times, and is less effective for metastatic cancer.
- Chemotherapy: Uses drugs to kill fast-dividing cancer cells and is effective for both localized and advanced stages. However, it affects both cancerous and healthy cells, causing side effects like fatigue, nausea, and immune suppression.
- Targeted Therapy: Specifically targets genetic mutations or proteins in cancer cells, allowing for a more precise approach with typically fewer side effects than chemotherapy. It’s effective in cancers with specific mutations, but cancer cells may develop resistance over time.
These therapies are often used in combination to maximize effectiveness, with each option tailored to the cancer’s stage, type, and the patient’s health needs.
You can Learn More About How Chemotherapy differs from Immunotherapy: Special Article by OncoDaily
What Are the Long-Term Effects of Immunotherapy?
Long-term effects of immunotherapy can include durable remission and prolonged immune vigilance against cancer, offering potential long-term cancer control. However, some patients may experience persistent side effects, such as fatigue, thyroid issues, or chronic inflammation in organs like the lungs or liver. Regular monitoring and follow-up care are essential to manage these ongoing effects and support overall health.
Can All Bladder Cancer Patients Receive Immunotherapy?
Eligibility for immunotherapy in bladder cancer patients depends on several factors:
- Bladder Cancer Type and Stage: Immunotherapy is typically recommended for advanced or metastatic bladder cancer, particularly if other treatments like chemotherapy have failed. Non-muscle invasive cases may also be eligible if they are unresponsive to BCG therapy.
- PD-L1 Expression: Testing for PD-L1 levels can help identify patients likely to benefit from certain immunotherapy drugs, as high PD-L1 expression is often associated with better responses to checkpoint inhibitors.
- Overall Health: Patients need a stable level of health to tolerate immunotherapy. Those with autoimmune disorders or poor organ function may face higher risks with immunotherapy, making careful evaluation essential.
- Previous Treatment Response: Immunotherapy is often considered for patients who did not respond well to chemotherapy or other treatments.
These criteria help ensure immunotherapy is targeted to patients most likely to benefit, maximizing both safety and effectiveness
How Can Patients Support Their Immune System During Treatment?
To support the immune system during immunotherapy for bladder cancer, patients can make a few focused lifestyle and dietary changes:
- Balanced Diet: Emphasize nutrient-rich foods, including a variety of fruits, vegetables, whole grains, and lean proteins. These support immune health and overall energy. Limiting processed foods and sugars is also beneficial.
- Stay Hydrated: Drinking enough water can help flush toxins from the body and alleviate some side effects of immunotherapy.
- Regular Light Exercise: Gentle activities, like walking or yoga, can improve immune function, boost mood, and reduce fatigue, as long as it’s manageable.
- Stress Management: Techniques like meditation, breathing exercises, and mindfulness help reduce stress, which can positively affect immune health.
- Supplements: Consult a healthcare provider before adding any supplements. Vitamin D, probiotics, and omega-3s are often recommended for immune support but should be used under guidance to avoid interactions.
These adjustments provide support to both body and mind, helping improve resilience and quality of life during immunotherapy.
What Research Is Being Done on Immunotherapy for Bladder Cancer?
Bladder cancer immunotherapy continues to evolve with groundbreaking research focusing on combination therapies and next-generation drugs to overcome treatment resistance and improve outcomes.
- Enfortumab Vedotin with Pembrolizumab: The KEYNOTE-869/EV-103 trial reported a remarkable 73% overall response rate, demonstrating enhanced efficacy in advanced bladder cancer patients. This combination offers hope for those unresponsive to standard therapies.
- Sacituzumab Govitecan: As a Trop-2 targeting antibody-drug conjugate, Sacituzumab Govitecan showed a 27% overall response rate in the TROPHY-U-01 trial, effectively reducing tumors in metastatic bladder cancer patients.
- Dual Checkpoint Inhibitors: Nivolumab combined with Ipilimumab is under investigation to deliver long-lasting immune responses in advanced-stage bladder cancer. Early data suggests this strategy may significantly enhance survival rates.
- Personalized Treatments: Ongoing studies explore tailored immunotherapies based on patients’ unique tumor profiles, including mRNA vaccines and precision medicine approaches.
FAQ
What Is the Role of Immunotherapy in Bladder Cancer Treatment?
Immunotherapy enhances the immune system’s ability to identify and destroy bladder cancer cells. It is particularly effective in advanced stages or in cases where chemotherapy has failed, offering patients new treatment options.
How Do Checkpoint Inhibitors Work in Bladder Cancer?
Checkpoint inhibitors block proteins like PD-1 or PD-L1, which cancer cells use to evade the immune system. By inhibiting these checkpoints, the immune system is reactivated to attack cancer cells effectively.
Can BCG Therapy Be Combined With Other Immunotherapies?
Yes, ongoing research explores combining BCG therapy with immune checkpoint inhibitors for non-muscle-invasive bladder cancer (NMIBC) to enhance the immune response and improve outcomes for patients unresponsive to BCG alone.
What Are the Most Common Side Effects of Immunotherapy for Bladder Cancer?
Patients often experience fatigue, nausea, and mild skin rashes. Serious side effects can include immune-related issues like inflammation in the lungs (pneumonitis) or liver (hepatitis), which require close monitoring.
How Does Immunotherapy Compare to Chemotherapy in Bladder Cancer?
Immunotherapy generally causes fewer systemic side effects than chemotherapy and can produce longer-lasting responses. However, it may not work for all patients, while chemotherapy remains effective for a broader population.
What Is the Success Rate of Immunotherapy for Advanced Bladder Cancer?
Success rates vary, but checkpoint inhibitors like pembrolizumab and nivolumab achieve overall response rates of 20-30% in advanced bladder cancer, with some patients experiencing durable remissions.
Is Immunotherapy Effective for Early-Stage Bladder Cancer?
Immunotherapy, particularly BCG, is highly effective for non-muscle-invasive bladder cancer (NMIBC), achieving remission rates of up to 70%. Research is expanding its use in early stages to improve outcomes further.
What Is Nadofaragene Firadenovec, and How Does It Work?
Nadofaragene Firadenovec (Adstiladrin) is a gene therapy that delivers the interferon alfa-2b gene into bladder cells, stimulating a strong local immune response. It is effective for BCG-unresponsive NMIBC, with a complete response rate of 53% at three months.
Are Clinical Trials Available for Immunotherapy in Bladder Cancer?
Yes, many trials are investigating novel combinations and approaches, such as dual checkpoint inhibitors, personalized therapies, and antibody-drug conjugates like Sacituzumab Govitecan, offering new hope for treatment-resistant cases.
How Can Patients Manage Side Effects During Immunotherapy?
Patients can manage side effects by staying hydrated, eating a balanced diet, practicing stress reduction techniques, and taking prescribed medications for specific symptoms. Regular check-ups help address any serious issues promptly.
Written by Toma Oganezova, MD