Trastuzumab deruxtecan (T-DXd), an antibody-drug conjugate, is widely used for treating HER2-positive and HER2-low solid tumors, showing promising efficacy across multiple cancer types. However, one of the significant risks associated with its use is interstitial lung disease/pneumonitis (ILD), a serious adverse event that may require treatment interruption. This pooled analysis examines the safety and outcomes of T-DXd retreatment in patients who have recovered from grade 1 ILD, with a focus on the duration of retreatment and the recurrence of ILD. By exploring data from nine clinical trials, the study provides valuable insights into the potential for continued therapeutic benefit while managing the risks of ILD.
Title: Pooled analysis of trastuzumab deruxtecan retreatment after recovery from grade 1 interstitial lung disease/pneumonitis
Authors: H.S. Rugo, E. Tokunaga, H. Iwata, V. Petry, E.F. Smit, Y. Goto, D.-W. Kim, K. Shitara, J. Franklin Gruden, S. Modi, J. Cortés, I. Krop, P.A. Jänne, Y. Cheng, C. Taitt, F.-C. Cheng, C.A. Powell
Published in Annals of Oncology, August 2025
Background
Trastuzumab deruxtecan (T-DXd) is a novel antibody-drug conjugate (ADC) designed to target HER2, a protein overexpressed in various cancers, including HER2-positive breast cancer, gastric cancer, and non-small cell lung cancer (NSCLC). It combines a humanized monoclonal antibody against HER2 with a potent chemotherapy drug, allowing for targeted delivery to tumor cells. T-DXd has shown significant efficacy in improving progression-free survival and overall survival in patients with HER2-positive or HER2-low solid tumors who have failed prior therapies.
However, its use is not without risks, as one of the most concerning adverse events is interstitial lung disease/pneumonitis (ILD), a potentially life-threatening condition that can lead to respiratory compromise.
In a previous pooled analysis of early T-DXd clinical trials across multiple tumor types, ILD was observed in 15.4% of patients (177 out of 1150), with fatal grade 5 ILD occurring in 2.2% (25 out of 1150) of cases. Of the reported ILD cases, 77.4% were classified as grade 1 (27.1% of patients, or 48/177) or grade 2 (50.3%, or 89/177). Grade 3 ILD occurred in 7.9% (14/177), grade 4 in 0.06% (1/177), and grade 5 ILD was noted in 14.1% (25/177) of the cases.
ILD encompasses a variety of lung disorders characterized by inflammation and fibrosis, and is a known side effect of several cancer therapies, including T-DXd. Common signs of ILD include abnormalities on chest imaging, reduced lung function (as seen in tests like diffusing capacity for carbon monoxide and forced expiratory volume), and symptoms such as dyspnea and cough.
The National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) classifies ILD severity as follows: grade 1 involves no symptoms with non-specific radiographic changes; grade 2 includes symptoms requiring medical intervention; grade 3 is characterized by severe symptoms and may require oxygen; grade 4 represents life-threatening conditions requiring urgent intervention, such as intubation; and grade 5 refers to fatal ILD. In 2017, an independent, multidisciplinary adjudication committee was formed to review ILD events in patients treated with T-DXd.
Although the exact clinical factors contributing to T-DXd-related ILD remain under study, a pooled analysis of patients with extensive prior therapy (3-10 prior treatment lines) from nine T-DXd monotherapy trials indicated that factors such as renal dysfunction, baseline hypoxia, age, and treatment in Japan could be linked to a higher incidence of ILD. The underlying mechanism of T-DXd-related ILD is still unclear, necessitating further investigation. According to the FDA and EMA guidelines for T-DXd, treatment should be interrupted if ILD is suspected, and corticosteroid therapy should be initiated. Treatment can resume for patients who experience grade 1 ILD once it resolves to grade 0, with a dose reduction if the resolution takes longer than 28 days.
However, T-DXd must be permanently discontinued for grade 2 or higher ILD. Despite these guidelines, there is limited data on the risk of ILD recurrence after T-DXd retreatment, and understanding these risks is crucial for optimizing the safety and efficacy of retreatment. This analysis combines data from nine phase 1-3 T-DXd trials to better characterize the outcomes of retreatment and the recurrence of ILD following recovery from grade 1 ILD.
Methods and Study design
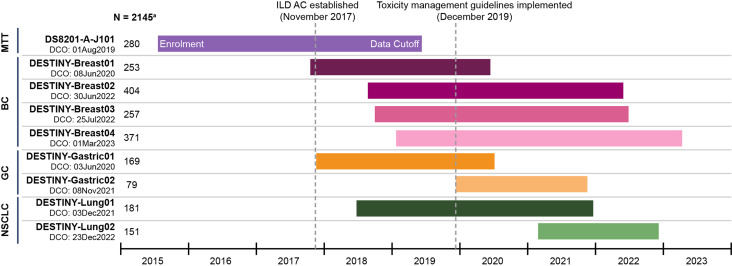
The data were pooled from nine clinical trials involving 2145 patients with HER2-positive or HER2-low breast cancer (BC), HER2-positive gastric cancer, HER2-positive non-small cell lung cancer (NSCLC), and other solid tumors. Patients who experienced grade 1 ILD (confirmed by both investigator and an independent adjudication committee) and recovered were eligible for T-DXd retreatment. The duration of retreatment and the recurrence of ILD were assessed until disease progression or the data cutoff.
The study included a population of 2,145 patients diagnosed with various HER2-positive or HER2-low solid tumors, all of whom were treated with trastuzumab deruxtecan (T-DXd). The patients received T-DXd doses ranging from 5.4 to 8.0 mg/kg, with adjustments made to the dose as necessary. Throughout the treatment period, patients were closely monitored for the recurrence of interstitial lung disease (ILD) and for any progression of their disease.
- Primary Endpoint: ILD recurrence after retreatment.
- Secondary Endpoints: Duration of retreatment, best response, and safety.
Results
The results showed that T-DXd retreatment after recovery from grade 1 ILD was generally safe, with a manageable rate of ILD recurrence. Most patients experienced mild or moderate recurrences, which were effectively addressed using existing treatment guidelines.
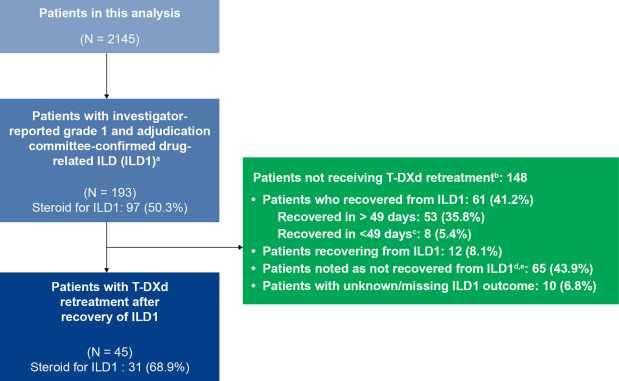
- Prevalence of Grade 1 ILD: 9% of patients (193/2145) experienced grade 1 ILD.
- Retreatment: 23.3% of ILD patients (45/193) were retreated with T-DXd.
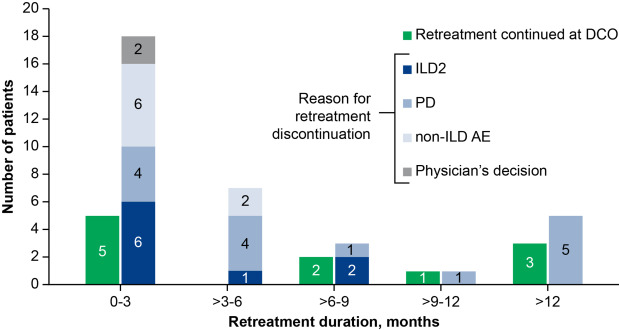
- Duration: The median retreatment duration was 85 days (range: 1-848 days).
- ILD Recurrence: 33.3% (15/45) of retreated patients experienced ILD recurrence. All recurrence events were either grade 1 or grade 2.
- Outcome: 60% of patients who experienced recurrent ILD (ILD2) recovered with or without sequelae.
- Discontinuations: 33.3% of patients discontinued due to disease progression, while 20% stopped because of ILD recurrence.
Key Findings
- Retreatment with T-DXd after grade 1 ILD recovery was associated with manageable risks, as all recurrent ILD events were low-grade.
- The overall response rate before retreatment was 82.2%, with most patients maintaining or improving their previous tumor response.
- ILD recurrence occurred in 33.3% of retreated patients, highlighting the importance of early monitoring and adherence to guidelines.
Key Takeaway Messages
- T-DXd retreatment after recovery from grade 1 ILD is a viable option for maximizing therapeutic benefit, provided there is careful monitoring.
- Recurrence of ILD, although common, remains manageable, with no grade 3 or higher events reported.
- Continuous follow-up, including regular imaging and steroid management, is crucial for minimizing risks associated with retreatment.
Conclusion
This pooled analysis confirms that T-DXd retreatment following the resolution of grade 1 ILD is generally safe, with manageable risks of ILD recurrence. While recurrence was noted in one-third of patients, the ILD events were predominantly low-grade and responded well to treatment. T-DXd retreatment has the potential to offer continued benefit for patients with metastatic or advanced solid tumors after recovering from grade 1 ILD, optimizing the therapeutic outcome when managed according to current safety guidelines.
You can read the full article here.
Written by Sona Karamyan,MD


