Trastuzumab deruxtecan (Enhertu) is a smart drug that targets HER2-positive cancer cells, delivering a potent chemotherapy payload directly to the tumor. The FDA first approved it in December 2019 for metastatic HER2-positive breast cancer and later expanded to gastric cancer (2021), HER2-low breast cancer (2022), and HER2-mutant lung cancer (2023). With impressive results in clinical trials, it’s redefining treatment for HER2-driven cancers.
Which company produced Trastuzumab deruxtecan?
Enhertu is a breakthrough cancer therapy developed by Daiichi Sankyo in collaboration with AstraZeneca. Daiichi Sankyo, a Japanese pharmaceutical company headquartered in Tokyo, was formed in 2005 through the merger of Daiichi Pharmaceutical and Sankyo Co. With a strong focus on oncology, cardiovascular diseases, and vaccines, it has pioneered advancements in antibody-drug conjugates (ADCs), including Enhertu. AstraZeneca, a British-Swedish pharmaceutical giant based in Cambridge, UK, is a global leader in oncology and other therapeutic areas. By partnering with Daiichi Sankyo, AstraZeneca helped bring Enhertu to a broader market, expanding access to this innovative HER2-targeted treatment worldwide.
How does Trastuzumab deruxtecan work?
In normal cells, the HER2 protein helps regulate growth, but in some cancers, it becomes overactive, driving uncontrolled tumor growth. Trastuzumab deruxtecan is designed to target these HER2-expressing cancer cells with precision. The trastuzumab component binds to HER2 receptors on the tumor, triggering the cell to internalize the drug. Once inside, the linker breaks, releasing deruxtecan, a potent chemotherapy agent that disrupts DNA replication and forces the cancer cell into programmed death. What makes Enhertu even more powerful is its bystander effect, where the released drug also kills nearby tumor cells, even those with lower HER2 expression, making it a game-changer in HER2-driven cancers.
What Cancers Is Ehertu Approved to Treat?
Trastuzumab deruxtecan is FDA-approved to treat several HER2-expressing and HER2-mutant cancers:
- HER2-Positive Breast Cancer – For metastatic or unresectable cases after prior anti-HER2 therapy.
- HER2-Low Breast Cancer – Expanding treatment to tumors with low HER2 expression (IHC 1+ or IHC 2+/ISH-).
- HER2-Positive Gastric or Gastroesophageal Junction (GEJ) Cancer – For metastatic cases after trastuzumab therapy.
- HER2-Mutant Non-Small Cell Lung Cancer (NSCLC) – For previously treated HER2-mutant NSCLC.
What research is behind the approval?
Trastuzumab deruxtecan received FDA approval based on several pivotal clinical trials demonstrating its efficacy and safety across various cancers. Below is a summary of key trials supporting its approved indications:
1. HER2-Positive Breast Cancer (December 2019 Approval)
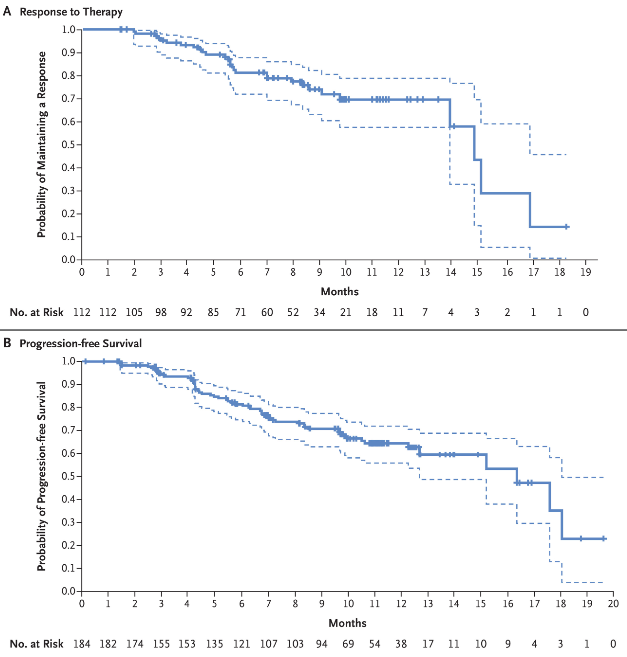
The pivotal DESTINY-Breast01 trial, published on December 11, 2019, in the New England Journal of Medicine (NEJM), confirmed the efficacy of Trastuzumab deruxtecan in HER2-positive metastatic breast cancer after prior treatment with trastuzumab emtansine. This phase 2 study enrolled 184 heavily pretreated patients (median of six prior therapies), demonstrating a 60.9% objective response rate (ORR) and a median progression-free survival of 16.4 months. The median response duration was 14.8 months. Common grade ≥3 adverse events included neutropenia (20.7%) and anemia (8.7%), while interstitial lung disease (ILD) occurred in 13.6% of patients, requiring careful monitoring. These findings established Enhertu as a potent treatment option for patients with HER2-positive metastatic breast cancer.
2. HER2-Positive Gastric or Gastroesophageal Junction (GEJ) Cancer (January 2021 Approval)
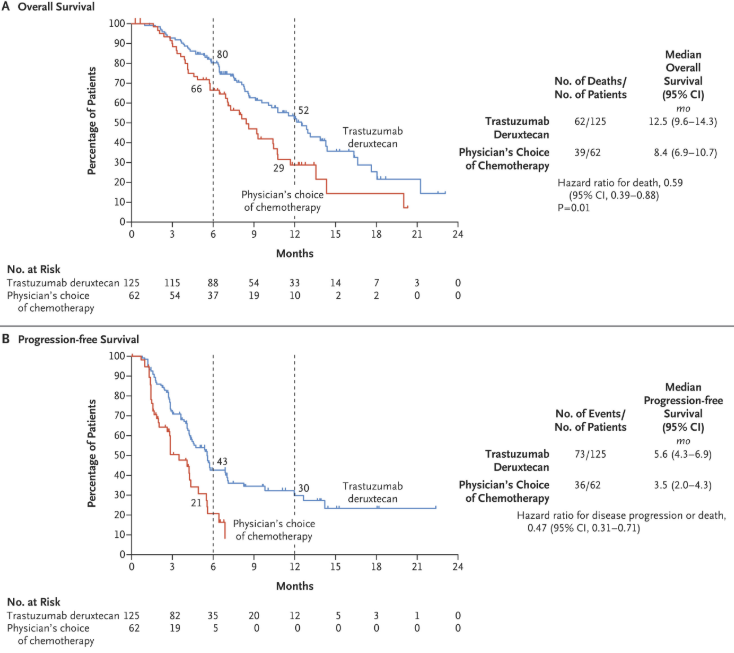
The DESTINY-Gastric01 trial, published in NEJM on May 29, 2020, demonstrated the efficacy of Trastuzumab deruxtecan in HER2-positive advanced gastric or gastroesophageal junction cancer after at least two prior treatments. Among 187 patients, the objective response rate was 51% with Enhertu versus 14% with chemotherapy (P<0.001), and overall survival improved (12.5 vs. 8.4 months, HR 0.59, P=0.01). Common grade ≥3 adverse events included neutropenia (51%) and anemia (38%), while interstitial lung disease occurred in 12 patients. These findings established Trastuzumab deruxtecan as a superior option for this patient group.
3. HER2-Low Breast Cancer (August 2022 Approval)
The DESTINY-Breast04 trial, published in NEJM on June 5, 2022, demonstrated the efficacy of trastuzumab deruxtecan in HER2-low metastatic breast cancer, a subgroup previously unresponsive to HER2-targeted therapy. Among 557 patients, Enhertu significantly improved progression-free survival (10.1 vs. 5.4 months, HR 0.51, P<0.001) and overall survival (23.9 vs. 17.5 months, HR 0.64, P=0.003) in hormone-receptor-positive patients. Similar benefits were seen in the overall cohort. Grade ≥3 adverse events were lower with Enhertu (52.6%) than with chemotherapy (67.4%), though 12.1% experienced interstitial lung disease. These results established Enhertu as the first effective HER2-targeted therapy for HER2-low breast cancer.

You can read more about Breast Cancer: Symptoms Causes, Stages, Diagnosis and Treatment on OncoDaily.
4. HER2-Mutant Non-Small Cell Lung Cancer (NSCLC) (April 2023 Approval)
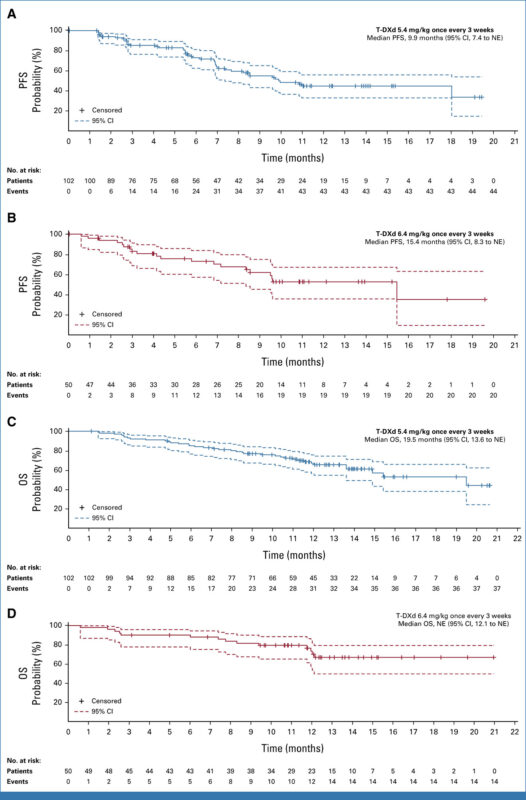
The DESTINY-Lung02 trial, published in JCO on September 11, 2023, evaluated Trastuzumab deruxtecan 5.4 mg/kg for the first time in HER2-mutant metastatic non-small cell lung cancer (mNSCLC) after platinum-based therapy. Among 152 patients, the objective response rate was 49.0% for 5.4 mg/kg and 56.0% for 6.4 mg/kg, with median response durations of 16.8 months and not estimable, respectively. Grade ≥3 adverse events were lower with 5.4 mg/kg (38.6%) than 6.4 mg/kg (58.0%), and interstitial lung disease occurred in 12.9% and 28.0% of patients, respectively. The 5.4 mg/kg dose was favored for its efficacy and better safety profile.
5. HER2-Positive Solid Tumors (April 2024 Accelerated Approval)
The DESTINY-PanTumor02 trial, published in JCO on October 23, 2023, showed trastuzumab deruxtecan 5.4 mg/kg had a 37.1% response rate in HER2-expressing solid tumors, with the highest benefit in HER2 IHC 3+ (61.3%). Median PFS was 6.9 months, OS 13.4 months. Safety was consistent with prior studies, including 10.5% interstitial lung disease (3 fatal cases), supporting Trastuzumab deruxtecan as a tumor-agnostic therapy.
These trials collectively underscore Trastuzumab deruxtecan‘s efficacy across a spectrum of HER2-expressing and HER2-mutant cancers, leading to its multiple FDA approvals.

Learn more about Stomach Cancer: Symptoms and Causes, Types, Diagnosis and Treatment on OncoDaily.
Combinations and Treatment Outcomes
DESTINY-Breast07 evaluated Trastuzumab deruxtecan (T-DXd) ± pertuzumab (P) as a first-line treatment for HER2+ metastatic breast cancer. Among 125 patients, the most common adverse event was nausea, and ILD rates were ≤10% (all Grade ≤2). Confirmed ORR was 77.3% (T-DXd) and 82.0% (T-DXd + P), with 12-month PFS rates of 77.3% and 89.4%. Safety was consistent with known profiles, and efficacy appeared promising. Published in JCO, May 29, 2024.
DESTINY-Breast08 evaluated Trastuzumab deruxtecan (T-DXd) with endocrine therapy (ET) in HER2-low, HR+ metastatic breast cancer. Among 41 patients, adverse events were manageable, with interstitial lung disease reported in 15% of the T-DXd + fulvestrant arm. Confirmed ORR was 71.4% (T-DXd + anastrozole) and 40.0% (T-DXd + fulvestrant). T-DXd-ET combinations showed promising activity. Published in Cancer Research, May 2, 2024.
DESTINY-Gastric03 evaluated Trastuzumab deruxtecan (T-DXd) with fluoropyrimidines in HER2+ gastric/gastroesophageal junction cancer. Among 25 patients, recommended phase 2 doses were established. The most common grade ≥3 adverse events included anemia and neutropenia. Preliminary ORR for T-DXd + capecitabine was 43% (confirmed + unconfirmed). Findings suggest feasibility and activity in heavily pretreated patients. Published in JCO, January 19, 2022.
DESTINY-Gastric02 evaluated Trastuzumab deruxtecan (T-DXd) in HER2+ gastric/gastroesophageal junction cancer in the USA and Europe. Among 79 patients, the confirmed objective response rate was 38% at the primary analysis and 42% at the updated analysis. Median progression-free survival was 5.6 months, and median overall survival was 12.1 months. Common grade ≥3 adverse events included anemia (14%) and nausea (8%). Published in The Lancet, July 2023.
Trastuzumab deruxtecan side effects and its management
Trastuzumab deruxtecan is an antibody-drug conjugate (ADC) used to treat HER2-expressing cancers, including breast, gastric, and lung cancers. While effective, it is associated with several side effects that require careful management.
Common Side Effects and Management
- Hematologic Toxicities (Neutropenia, Anemia, Thrombocytopenia)
Management: Routine blood tests are recommended to monitor blood counts. Growth factor support (e.g., G-CSF) may be required for neutropenia. Red blood cell transfusions or iron supplementation can help manage anemia. - Gastrointestinal Symptoms (Nausea, Vomiting, Diarrhea, Constipation)
Management: Prophylactic antiemetics (e.g., ondansetron) can help with nausea and vomiting. Adequate hydration and dietary adjustments are essential. Antidiarrheal agents like loperamide can be used if needed. - Fatigue
Management: Patients should be advised to balance activity with rest. Adjusting schedules, incorporating light exercise, and addressing any underlying anemia may help improve energy levels.
Less Common but Serious Side Effects and Management
- Interstitial Lung Disease (ILD) /Pneumonitis
Management: Patients should be monitored for symptoms such as cough, shortness of breath, or fever. If ILD is suspected, immediate imaging (e.g., CT scan) should be performed, and corticosteroids should be initiated. T-DXd should be permanently discontinued in severe cases. - Cardiac Toxicity (Left Ventricular Dysfunction, Heart Failure)
Management: Baseline and periodic echocardiograms are recommended to monitor heart function. If a decline in LVEF is detected, T-DXd should be withheld, and a cardiology consultation should be considered. - Infusion-Related Reactions
Management: Premedication with antihistamines or corticosteroids may help reduce the risk of reactions. Slowing or pausing the infusion can be considered if mild symptoms occur.
What is the Recommended Dosage of Trastuzumab deruxtecan?
Trastuzumab deruxtecan, available as a 100 mg/vial IV infusion, is used for HER2-expressing cancers. In breast cancer (HER2-positive, HER2-low, and HR+/HER2-ultralow), it is given at 5.4 mg/kg every three weeks following prior therapy. For HER2-mutant non-small cell lung cancer (NSCLC), the same dosage applies after systemic treatment. Patients with HER2-positive gastric or gastroesophageal junction (GEJ) cancer receive 6.4 mg/kg every three weeks after trastuzumab. In HER2-positive solid tumors, T-DXd is an option when no other treatments are available, administered at 5.4 mg/kg every three weeks. Treatment continues until disease progression or unacceptable toxicity.
How is Trastuzumab deruxtecan administered?
Trastuzumab deruxtecan is incompatible with 0.9% NaCl but compatible with Dextrose 5%. Verify vial labels before preparation. Reconstitute immediately with 5 mL sterile water per 100 mg vial (final concentration: 20 mg/mL). Swirl gently, do not shake, and discard if cloudy.
Dilute in 100 mL D5W, mix gently, and protect from light. Premedicate with antiemetics.
Administer IV infusion only with a 0.20–0.22 micron filter. Do not give as IV push/bolus or mix with other drugs. First dose: 90 min; subsequent: 30 min if tolerated. Slow or stop for reactions; discontinue if severe.
For missed doses, give ASAP while maintaining a 3-week interval. Store vials refrigerated (2-8°C), protected from light. Use reconstituted vials within 24 hours. Diluted solutions last 4 hours at room temp or 24 hours refrigerated. Do not freeze, shake, or expose to light.
What to Avoid During Trastuzumab deruxtecan Treatment?
To ensure safe and effective Trastuzumab deruxtecan treatment for HER2-positive cancer, avoid live vaccines due to infection risk and consult your doctor before vaccination. Pregnancy and breastfeeding are not recommended, so contraception is essential. Certain medications, especially CYP3A inhibitors or inducers, may interfere with Trastuzumab deruxtecan—always check with your doctor before taking new drugs.
Do not mix Trastuzumab deruxtecan with other IV medications, and store it properly, avoiding shaking or freezing. If you experience fatigue or dizziness, avoid driving or operating machinery. Staying on the 3-week dosing schedule is crucial; if a dose is missed, take it as soon as possible.
Following these precautions helps ensure a safer and more effective treatment.
Trastuzumab deruxtecan deffectiveness over time
Trastuzumab deruxtecan remains effective in treating HER2-positive cancers, but its effectiveness can change over time due to disease progression, acquired resistance, or tolerability issues.
Initially, Trastuzumab deruxtecan shows high response rates, especially in patients with previously treated metastatic HER2-positive breast or gastric cancer. However, some tumors may develop resistance through mechanisms like HER2 loss, changes in drug metabolism, or alterations in DNA damage repair pathways.
The duration of response varies, with clinical trials showing median progression-free survival (PFS) ranging from several months to over a year, depending on cancer type and prior treatments. Effectiveness may decline with prolonged use, requiring treatment adjustments or switching to alternative therapies.
Regular monitoring through imaging and biomarker assessments helps evaluate response and guide treatment decisions. If disease progression occurs, oncologists may explore combination therapies, alternative HER2-targeted agents, or clinical trials.
Ongoing trials with Trastuzumab deruxtecan
The DO-01 (NCT06819007) trial is a Phase 3 study evaluating trastuzumab deruxtecan + bevacizumab versus bevacizumab alone as first-line maintenance in HER2-expressing advanced ovarian cancer. A safety run-in phase will precede randomization. Known as DESTINY-Ovarian01, this trial assesses whether T-DXd improves outcomes over bevacizumab alone.
The DESTINY-Gastric05 (NCT06731478) trial is a Phase 3 study comparing Trastuzumab deruxtecan + chemotherapy + pembrolizumab to standard chemotherapy + trastuzumab + pembrolizumab as first-line treatment for unresectable or metastatic HER2-positive gastric or GEJ cancer with PD-L1 CPS ≥1. An exploratory cohort will assess T-DXd + chemotherapy versus standard therapy in PD-L1 CPS <1 patients.
The DISCORDANT (NCT06585969) trial is a Phase 3, international, randomized study comparing trastuzumab deruxtecan to CDK4/6 inhibitors with endocrine therapy as first-line treatment for non-Luminal A, ER-positive/HER2-low metastatic breast cancer. Patients receive either T-DXd (5.4 mg/kg IV every 21 days) until progression or a CDK4/6 inhibitor (Ribociclib or Abemaciclib) with endocrine therapy until progression. Radiological evaluations occur every 9–12 weeks.
The NCT06364410 trial is a Phase I study evaluating the safety, side effects, and optimal dose of azenosertib (Wee1 inhibitor) with trastuzumab deruxtecan in patients with HER2-positive, cyclin E-amplified gastric, gastroesophageal junction, or other solid tumors that are locally advanced, metastatic, or unresectable. This combination aims to enhance tumor response by making cancer cells more vulnerable to treatment.
What is a Tumor-Agnostic Drug and How Does It Work Across Different Cancers?
A drug used for different cancers means it targets a common feature, like a specific molecular marker, that is shared across various types of tumors. This approach allows a single drug to be effective against multiple cancers with similar characteristics, regardless of their location in the body.
In 2024, AstraZeneca announced that Enhertu has been approved by the FDA for adult patients with unresectable or metastatic HER2-positive solid tumors that have not responded to previous treatments. This approval is based on promising results from several Phase II trials, showing significant tumor response in multiple cancer types. As the first HER2-targeted antibody-drug conjugate with tumor-agnostic approval, Enhertu offers a new treatment option for cancers with HER2 overexpression, such as breast, lung, and colorectal cancers. The drug’s approval highlights the growing importance of targeting specific molecular markers in cancer treatment.
Written by Mariam Khachatryan, MD
FAQ
What is Enhertu used for?
Enhertu is approved for HER2-positive breast cancer, gastric cancer, and non-small cell lung cancer. It is also being studied for other HER2-expressing and HER2-mutant solid tumors.
How does Trastuzumab deruxtecan differ from other HER2-targeted therapies?
Trastuzumab deruxtecan is an antibody-drug conjugate (ADC) that combines a HER2-targeting antibody with a potent chemotherapy drug. This design allows it to deliver treatment directly to cancer cells while minimizing damage to healthy cells.
What is the recommended dosage of Enhertu?
The standard dose is 5.4 mg/kg every three weeks for breast and lung cancer, and 6.4 mg/kg every three weeks for gastric cancer. However, dosage may be adjusted based on tolerability and side effects.
How is Trastuzumab deruxtecan administered?
Enhertu is given as an intravenous (IV) infusion. The first infusion lasts 90 minutes, with subsequent doses given over 30 minutes if well tolerated.
What are the most common side effects of Enhertu?
Patients may experience nausea, fatigue, decreased appetite, low blood counts, and lung-related side effects such as interstitial lung disease (ILD). Side effect management is an essential part of treatment.
What precautions should patients take while on Enhertu?
Patients should undergo regular lung function and blood tests to monitor for toxicity. It's also crucial to report any new or worsening respiratory symptoms immediately.