Patients with high-risk locally advanced cutaneous squamous cell carcinoma (LA cSCC) face substantial risk of recurrence and metastasis, even after standard therapy with surgical resection and adjuvant radiotherapy (RT). Although PD-1 inhibitors such as pembrolizumab are approved for patients with unresectable or metastatic cSCC, their benefit in the adjuvant setting has remained unclear. The KEYNOTE-630 trial (NCT03833167) was designed to address this question by evaluating whether the addition of adjuvant pembrolizumab improves outcomes in patients with high-risk, resected LA cSCC following surgery and RT.
Study Design
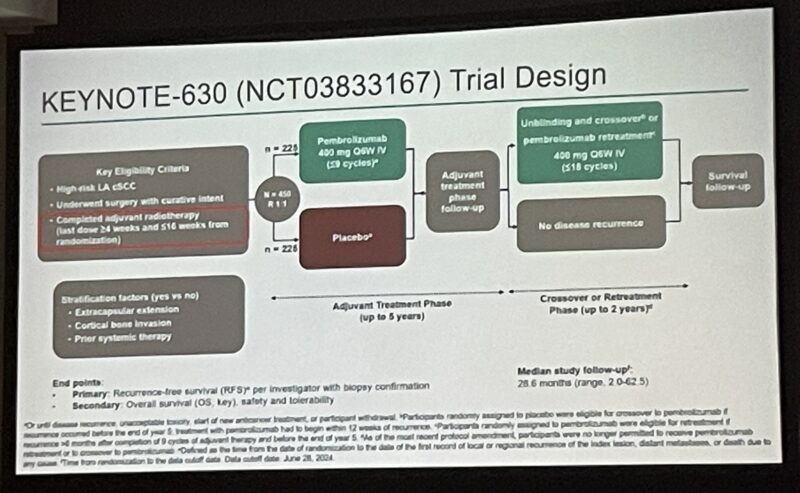
KEYNOTE-630 was a randomized, double-blind, phase 3 trial enrolling 450 adults with histologically confirmed LA cSCC and at least one protocol-defined high-risk feature. All participants underwent complete surgical resection and adjuvant RT, then were randomized to receive either pembrolizumab 400 mg IV or placebo every six weeks for up to nine cycles. The primary endpoint was recurrence-free survival (RFS); secondary endpoints included overall survival (OS) and safety.
Results
After a median follow-up of 28.6 months, the 24-month RFS rate was 78.3% for pembrolizumab versus 68.6% for placebo (hazard ratio [HR] 0.76, 95% CI 0.53–1.10; P = 0.07243). This difference did not meet the predefined boundary for statistical significance (p < 0.0160). Subgroup analyses indicated that patients with extracapsular extension (HR 0.44), those aged ≥65 years (HR 0.61), and non-smokers (HR 0.58) appeared to derive greater benefit from pembrolizumab. Locoregional recurrence was reduced (13.8% vs 25.3%), as was distant metastasis (4.4% vs 11.6%), but these improvements did not translate into a statistically significant RFS benefit for the entire study population.
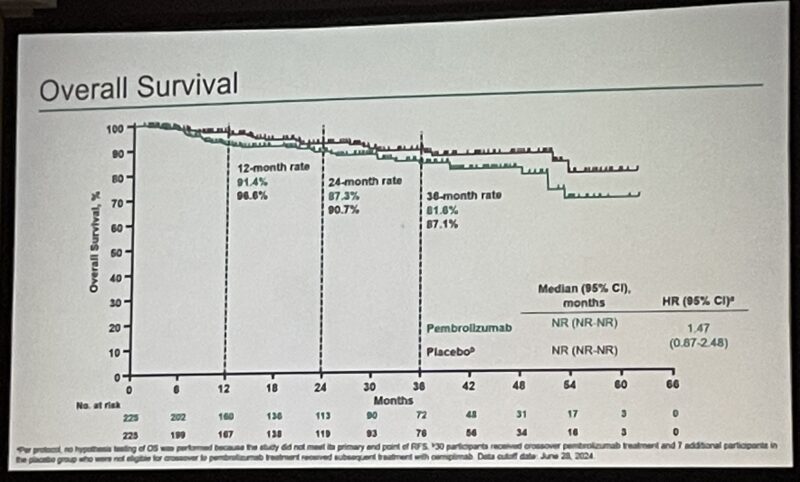
The 24-month OS rate was similar between groups: 87.3% for pembrolizumab and 90.7% for placebo (HR 1.47, 95% CI 0.87–2.48). Treatment-related adverse events (TRAEs) were more frequent in the pembrolizumab arm (63.8% vs 41.1%), including grade 3–4 events (7.6% vs 2.7%). No deaths were attributed to study drug, and discontinuations due to TRAEs were uncommon.
Conclusions
The KEYNOTE-630 trial demonstrated that adjuvant pembrolizumab, when given after surgery and RT in patients with high-risk resected LA cSCC, did not significantly improve recurrence-free survival compared to placebo. Although certain subgroups may have benefited, the overall lack of statistically significant improvement and the safety profile led to early study termination for futility on the recommendation of the data monitoring committee.
Clinical Implications
For patients with high-risk, resected LA cSCC, adjuvant pembrolizumab cannot be recommended as standard of care based on current evidence. The findings reinforce the need for further research to identify biomarkers or subpopulations that might benefit from immunotherapy in the adjuvant setting.
What They’re Saying: Reactions to KEYNOTE-630 Trial at ASCO 2025
C. Jillian Tsai, MD, PhD, Lead, Allan and Ruth Kerbel Palliative RT & Oligomet Program (PROP) shared on X
Adjuvant pembro after surgery and PORT for up to 9 cycles in high-risk locally advanced cutaneous squamous cell carcinoma did not improve RFS/OS.
Key Takeaway Messages from KEYNOTE-630 Trial
Study Objective: KEYNOTE-630 assessed adjuvant pembrolizumab for patients with high-risk, locally advanced cutaneous squamous cell carcinoma (cSCC) following surgery and radiation therapy.
Primary Outcome (Recurrence-Free Survival): The trial did not meet its primary endpoint, as pembrolizumab failed to significantly improve recurrence-free survival (RFS) compared to placebo (24-month RFS: 78.3% pembrolizumab vs. 68.6% placebo; HR 0.76, P = 0.0724).
Subgroup Findings: Despite overall negative results, specific subgroups benefited notably:
• Patients with extracapsular extension (HR: 0.44)
• Patients aged ≥65 years (HR: 0.61)
• Non-smokers (HR: 0.58)
Recurrence Patterns: Pembrolizumab reduced rates of local-regional recurrence (13.8% vs. 25.3%) and distant metastases (4.4% vs. 11.6%) compared to placebo.
Overall Survival: No significant difference in overall survival at 24 months was seen (Pembrolizumab: 87.3%, Placebo: 90.7%, HR 1.47).
Safety Profile: Pembrolizumab had a manageable and expected safety profile, with higher incidence of grade 3–4 treatment-related adverse events (7.6% pembrolizumab vs. 2.7% placebo), but no new safety signals or deaths from treatment-related toxicity.
Clinical Implication: Despite a lack of overall statistical significance, observed benefits in selected high-risk subgroups highlight the importance of further research into targeted patient populations for adjuvant immunotherapy in locally advanced cSCC.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


