Muscle-invasive bladder cancer (MIBC) remains a high-risk disease with a substantial rate of recurrence despite aggressive management. Platinum-based neoadjuvant chemotherapy (NAC) followed by radical cystectomy (RC) is a standard of care, but the long-term outcomes are suboptimal for many patients. The NIAGARA phase 3 trial (NCT03732677) recently demonstrated that adding perioperative durvalumab (D), an anti–PD-L1 antibody, to standard NAC results in significantly improved event-free survival (EFS), overall survival (OS), and a higher pathologic complete response (pCR) rate versus NAC alone in cisplatin-eligible MIBC. The current analysis explores circulating tumor DNA (ctDNA) dynamics as a biomarker of response and its association with clinical outcomes in NIAGARA.
Study Design and Methods
The NIAGARA study enrolled patients with histologically confirmed, cisplatin-eligible MIBC (clinical stage T2–T4aN0/1M0), all planned for radical cystectomy. Patients were randomized 1:1 to two arms:
- D Arm: Neoadjuvant durvalumab (1500 mg IV every 3 weeks) plus cisplatin and gemcitabine for 4 cycles, followed by RC, then adjuvant durvalumab monotherapy (1500 mg IV every 4 weeks) for 8 cycles.
- Comparator Arm (C): NAC with cisplatin and gemcitabine for 4 cycles, followed by RC alone
Dual primary endpoints included pathologic complete response (pCR) and event-free survival (EFS), while disease-free survival (DFS) served as a secondary endpoint. Plasma ctDNA was assessed using a personalized, tumor-informed molecular residual disease assay (Signatera MRD, Natera Inc.), at three timepoints: baseline (prior to NAC), post-NAC/pre-RC, and post-RC (at start of adjuvant phase).
Results
Of 1,063 patients randomized, 462 (237 in D arm; 225 in C arm) comprised the biomarker-evaluable population. Baseline characteristics matched the intention-to-treat cohort.
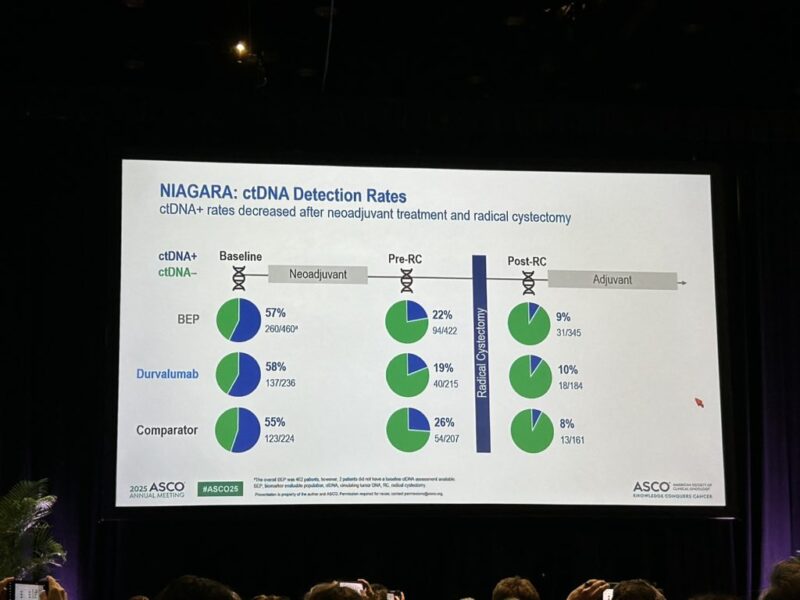
- ctDNA+ Rate Dynamics: At baseline, ctDNA was positive in 57% (260/460) of patients. After neoadjuvant treatment, prior to RC, the ctDNA+ rate decreased to 22% (94/422).
- ctDNA Clearance: ctDNA clearance from baseline to pre-RC was 41% in the D arm versus 31% in the C arm, indicating a greater proportion of patients achieving molecular response with perioperative durvalumab.
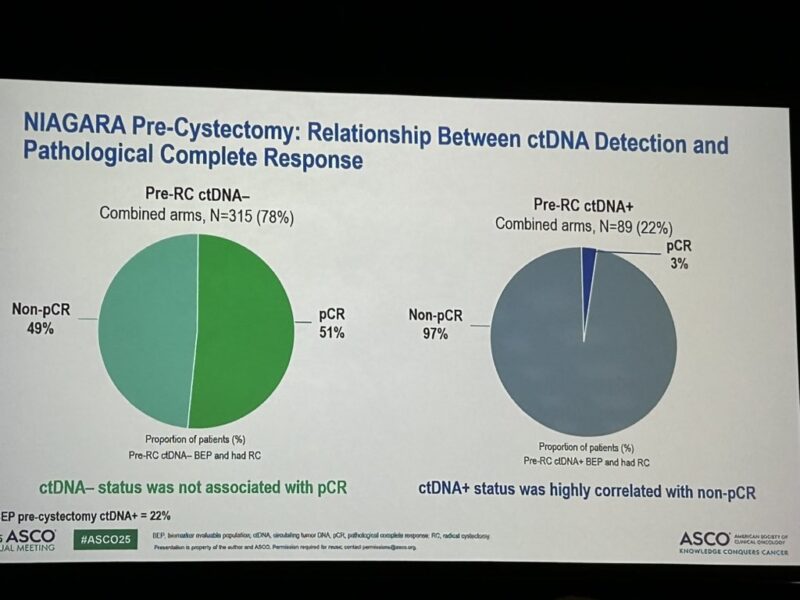
- Association with Pathologic Response: Among patients with pre-RC ctDNA+ status, 97% (86/89) did not achieve pCR, supporting ctDNA as a negative predictor for pathologic response.
- Post-RC ctDNA: The overall ctDNA+ rate post-RC was just 9% (31/345), further suggesting effective tumor clearance.
- Survival Outcomes by ctDNA Status: EFS benefit in the D arm versus C arm was evident for both baseline ctDNA+ (HR 0.73; 95% CI, 0.51–1.06) and ctDNA− groups (HR 0.45; 0.25–0.84). DFS benefit was also maintained after RC, in both ctDNA+ and ctDNA− groups (HR 0.49 for post-RC ctDNA+).
Interpretation
This analysis demonstrates that perioperative durvalumab, when combined with platinum-based NAC, not only improves classic clinical outcomes but also enhances molecular response as indicated by ctDNA clearance. Persistent ctDNA positivity after neoadjuvant therapy is strongly associated with failure to achieve pCR and may signal higher relapse risk. Conversely, effective ctDNA clearance correlates with improved EFS and DFS, regardless of baseline molecular status.
Clinical Implications
These findings position ctDNA as a promising, non-invasive biomarker for early response evaluation and relapse risk stratification in MIBC. The ability of perioperative durvalumab to increase ctDNA clearance rates supports its additional benefit over NAC alone. Integrating ctDNA testing could further personalize treatment and surveillance strategies for high-risk bladder cancer.
Conclusion
The NIAGARA trial’s exploratory ctDNA analysis confirms that perioperative durvalumab plus neoadjuvant chemotherapy provides substantial clinical and molecular benefit for cisplatin-eligible MIBC. Higher ctDNA clearance, improved EFS, and sustained DFS reinforce perioperative immunotherapy as a new therapeutic standard for these patients. These results underscore the utility of ctDNA to guide risk-adapted management and future research.
What They’re Saying: Reactions to TROPION-Lung02 at ASCO 2025
Dra. María Natalia Gandur Quiroga, Leader GU InstitutoRoffo shared on X
ctDNA+ rate: 57% (baseline) → 22% (pre-RC) → 9% (post-RC)
Pre-RC ctDNA+ strongly correlated with non-pCR (97%)
ctDNA clearance: 41% (D+NAC) vs 31% (NAC)
EFS benefit of periop. D seen in ctDNA+ & ctDNA− pts
DFS: ctDNA− vs ctDNA+ → HR 0.09
EFS: clearance vs persistent ctDNA+ → HR 0.32
DFS: ctDNA+ pCR vs non-pCR → HR 0.41
ctDNA is a strong prognostic biomarker. Periop. D improves outcomes regardless of ctDNA status.
Key Takeaways from the NIAGARA Trial ctDNA Analysis
The exploratory ctDNA analysis from the NIAGARA Phase 3 trial highlights that adding perioperative durvalumab to standard neoadjuvant chemotherapy in cisplatin-eligible muscle-invasive bladder cancer significantly enhances molecular response rates, as evidenced by higher ctDNA clearance compared to chemotherapy alone. Persistent ctDNA positivity after neoadjuvant treatment was strongly associated with failure to achieve pathologic complete response, underscoring ctDNA’s utility as a prognostic biomarker. Importantly, perioperative durvalumab improved event-free survival and disease-free survival across both ctDNA-positive and ctDNA-negative subgroups at baseline, and these benefits were also observed in patients who remained ctDNA-positive after surgery. Collectively, these findings reinforce the value of perioperative immunotherapy for high-risk bladder cancer and support the integration of ctDNA monitoring into future personalized treatment strategies.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


