What was the Background and Rationale for the BREAKWATER Trial?
BREAKWATER (NCT04607421) is a global, open-label, randomized phase 3 study evaluating first-line (1L) treatment with encorafenib plus cetuximab (EC) with or without chemotherapy (mFOLFOX6) versus standard of care (SOC; chemotherapy with or without bevacizumab) in patients with BRAF V600E-mutant metastatic colorectal cancer (mCRC). The study previously demonstrated a significant improvement in objective response rate (ORR) with EC+mFOLFOX6, leading to FDA accelerated approval under Project Frontrunner. This report presents the primary analysis of progression-free survival (PFS), updated overall survival (OS), safety, and other relevant outcomes.

How was the Study Designed and Who were the Patients?
Eligible patients with untreated BRAF V600E-mutant mCRC were randomized 1:1:1 to receive:
- EC alone,
- EC plus mFOLFOX6, or
- Standard of care (SOC).
Enrollment to the EC monotherapy arm was closed after a protocol amendment. The dual primary endpoints were ORR and PFS by blinded independent central review (BICR) comparing EC+mFOLFOX6 versus SOC. Overall survival (OS) was a key secondary endpoint.
What were the Key Results of the Trial?
A total of 637 patients were randomized across the three arms. Baseline characteristics were balanced. At data cutoff (January 6, 2025):
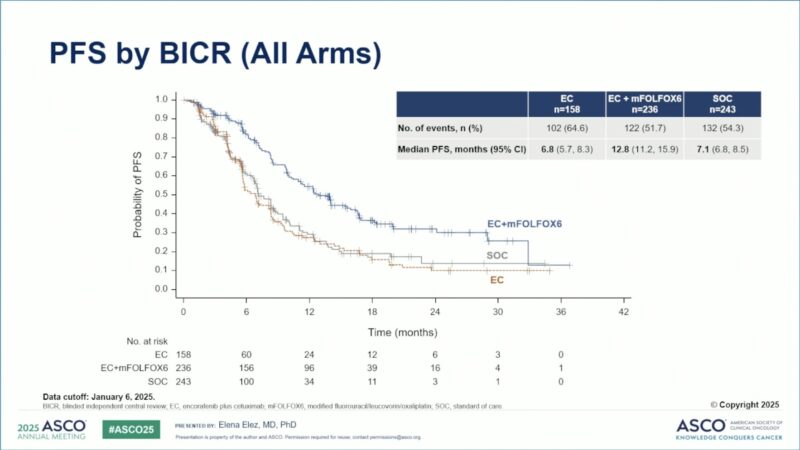
Progression-Free Survival (PFS):
- Median PFS was 12.8 months (95% CI 11.2–15.9) with EC+mFOLFOX6 versus 7.1 months (95% CI 6.8–8.5) with SOC (HR 0.53; 95% CI 0.41–0.68; P<0.0001).
- Median PFS for EC monotherapy was 6.8 months.
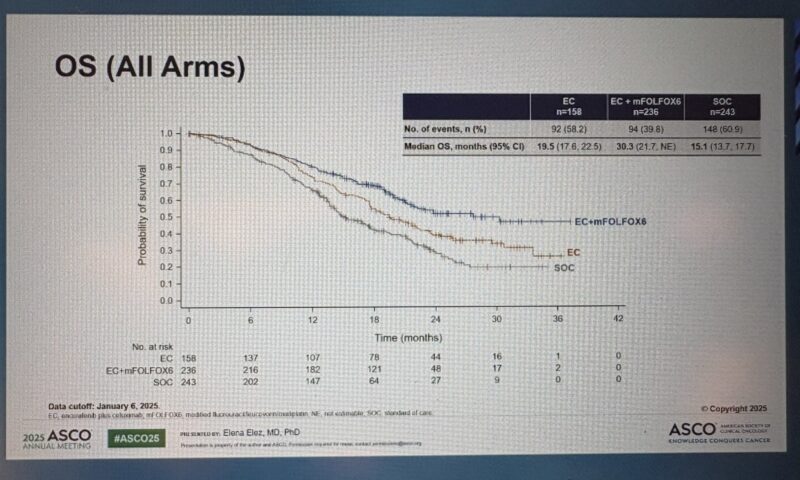
Overall Survival (OS):
Median OS was 30.3 months (95% CI 21.7–not estimable) with EC+mFOLFOX6 versus 15.1 months (95% CI 13.7–17.7) with SOC (HR 0.49; 95% CI 0.38–0.63; P<0.0001).
- Median OS for EC monotherapy was 19.5 months.
- Objective Response Rate (ORR):
65.7% (95% CI 59.4–71.4) with EC+mFOLFOX6,
37.4% (95% CI 31.6–43.7) with SOC,
45.6% (95% CI 38.0–53.3) with EC monotherapy.
Duration of Response (DOR):
Median DOR was 13.9 months with EC+mFOLFOX6, 10.8 months with SOC, and 7.0 months with EC monotherapy.
Safety:
- Serious treatment-emergent adverse events occurred in 30%, 46%, and 39% of patients in the EC, EC+mFOLFOX6, and SOC arms, respectively.
- Safety profiles were consistent with known effects of the agents.
Endpoint EC (n=158) EC+mFOLFOX6 (n=236) SOC (n=243) HR (95% CI) P-value
Median PFS (months) 6.8 (5.7–8.3) 12.8 (11.2–15.9) 7.1 (6.8–8.5) 0.53 (0.41–0.68) <0.0001
Median OS (months) 19.5 (17.6–22.5) 30.3 (21.7–NE) 15.1 (13.7–17.7) 0.49 (0.38–0.63) <0.0001
ORR (%) 45.6 65.7 37.4 — —
Median DOR (months) 7.0 (4.2–11.6) 13.9 (10.9–18.5) 10.8 (7.6–13.4)
What Conclusions were Drawn from the Study?
The BREAKWATER trial demonstrated that EC combined with mFOLFOX6 significantly improves progression-free and overall survival compared to standard chemotherapy regimens in first-line treatment of BRAF V600E-mutant mCRC. The combination showed a manageable safety profile and represents a potential new standard of care in this patient population.
And the fun starts! #asco25 press release Overall survival was 30.3 months in the encorafenib/cetuximab with mFOLFOX6 arm, 19.5 months in the encorafenib/cetuximab alone arm, and 15.1 months in the control arm.
From Dr. Gilberto Lopes’s X
Dr. Elez updated results of BREAKWATER #1L #BRAFV600E MT #mCRC PFS 12.8 mos/OS 30.3 mos FOLFOX-EC vs PFS 7. 1 mos/OS 15.1 mos SOC, impressive OS benefit even w/EC access in control arm. Activity shown for EC alone arm as well #ASCO25
From Dr. Jun Gong’s X
BREAKWATER OS results are in—and they should reshape the first-line standard for BRAF V600E-mutant mCRC. Encorafenib + cetuximab + mFOLFOX6 vs standard chemo ± bevacizumab: PFS: 12.8 vs 7.1 months (HR 0.53, p < 0.0001)
From Dr. Maroun Bow Zerdan’s X
You Can Also Read KANDLELIT-001 Trial Demonstrates Promising Activity of MK-1084 in KRAS G12C–Mutant Colorectal Cancer at ASCO 2025 by Oncodaily
Authors
Elena Elez, Takayuki Yoshino, Lin Shen, Sara Lonardi, Eric Van Cutsem, Cathy Eng, Tae Won Kim, Harpreet S. Wasan, Jayesh Desai, Fortunato Ciardiello, Rona Yaeger, Timothy S. Maughan, Van K. Morris II, Christina Wu, Tiziana Usari, Robert J. Laliberte, Samuel S. Dychter, Xiaosong Zhang, Josep Tabernero, Scott Kopetz
Organizations
Department of Medical Oncology, Vall d’Hebron University Hospital (HUVH), Vall d’Hebron Institute of Oncology (VHIO), IOB-Quiron, UVic-UCC, Barcelona, Spain; Department of Gastrointestinal Oncology, National Cancer Center Hospital East, Kashiwa, Japan; Beijing Cancer Hospital, Beijing, China; Veneto Institute of Oncology IOV, IRCCS, Padua, Italy; University Hospitals Gasthuisberg and University of Leuven (KU Leuven), Leuven, Belgium; Vanderbilt-Ingram Cancer Center, Nashville, TN; Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea; Hammersmith Hospital, Imperial College London, London, United Kingdom;
Peter MacCallum Cancer Centre and the University of Melbourne, Melbourne, VIC, Australia; Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy; Memorial Sloan Kettering Cancer Center, New York, NY; University of Liverpool, Liverpool, United Kingdom; Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX; Department of Hematology and Oncology, Mayo Clinic Arizona, Phoenix, AZ; Pfizer, Inc., Milan, Italy; Pfizer, Cambridge, MA; Pfizer, La Jolla, CA; Pfizer, South San Francisco, CA; Vall d’Hebron Hospital Campus and Institute of Oncology (VHIO), Barcelona, Spain; The University of Texas MD Anderson Cancer Center, Houston, TX.
Written by Aharon Tsaturyan MD


