Updated results from the ongoing Phase 1 KANDLELIT-001 trial were presented at the 2025 ASCO Annual Meeting. The study evaluated MK-1084—a next-generation, selective KRAS G12C inhibitor—both as monotherapy and in combination with cetuximab, with or without mFOLFOX6, in patients with advanced KRAS G12C–mutant colorectal cancer (CRC).
What Is the KANDLELIT-001 Trial?
KANDLELIT-001 is a first-in-human, multicenter Phase 1 trial investigating MK-1084, a potent KRAS G12C-GDP covalent inhibitor, in patients with advanced solid tumors harboring the KRAS G12C mutation. KRAS G12C is a common oncogenic driver in multiple cancers, particularly CRC and non-small cell lung cancer (NSCLC), and remains an area of high unmet clinical need.
This multi-arm study evaluated:
- MK-1084 monotherapy (n=53)
- MK-1084 + cetuximab (n=39)
- MK-1084 + cetuximab + mFOLFOX6 (n=29)
This approach allowed researchers to assess how MK-1084 performs alone and in combination settings, helping to define the optimal regimen for KRAS G12C–mutant cancers while closely monitoring safety, tolerability, and potential therapeutic benefit.
Trial Design and Objectives
Eligible patients had confirmed KRAS G12C mutations, measurable disease per RECIST v1.1, and an ECOG performance status of 0–1.
- Monotherapy arms: patients with solid tumors, including CRC, received MK-1084 once or twice daily at doses from 25–800 mg.
- Combination arm: patients with advanced CRC and 1–2 prior lines of therapy received MK-1084 plus cetuximab.
- Triplet arm: patients with CRC and 0–1 prior therapies received MK-1084 + cetuximab + mFOLFOX6.
The primary endpoints were safety (DLTs, AEs, and discontinuations). The key secondary endpoint was ORR by investigator assessment.
Efficacy Results
Efficacy outcomes were promising across all treatment arms, demonstrating meaningful tumor responses in this difficult-to-treat population.
- MK-1084 Monotherapy (n=53) showed a confirmed objective response rate (ORR) of 38% (95% CI: 25–52) and an unconfirmed ORR of 43% (95% CI: 30–58), with a median follow-up of 23.2 months.
- MK-1084 + Cetuximab (n=39) improved confirmed ORR to 46% (95% CI: 30–63) and unconfirmed ORR to 56% (95% CI: 40–72), with a median follow-up of 9.2 months.
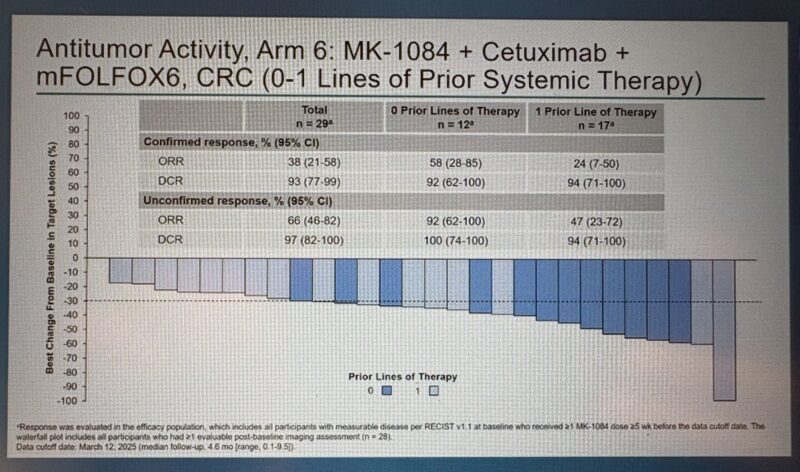
- MK-1084 + Cetuximab + mFOLFOX6 (n=29) achieved a confirmed ORR of 38% (95% CI: 21–58) and the highest unconfirmed ORR of 66% (95% CI: 46–82), despite a shorter median follow-up of 4.6 months.
All responses were partial responses, with no complete responses reported at this stage.
Safety and Tolerability
MK-1084 was generally well tolerated across all regimens. The combination therapies showed higher rates of treatment-related adverse events (TRAEs) but maintained manageable safety profiles.
- TRAEs were reported in 95% of patients receiving MK-1084 plus cetuximab and in 97% of patients treated with the triplet combination including chemotherapy.
- The most common adverse events included rash, diarrhea, fatigue, nausea, and elevated liver enzymes.
- The safety profile was consistent with expectations for the individual agents, and no new safety signals emerged during the study.
Key Takeaways
The updated results from the KANDLELIT-001 trial highlight the potential of MK-1084, a next-generation KRAS G12C-GDP covalent inhibitor, as a promising therapeutic option for patients with advanced colorectal cancer harboring KRAS G12C mutations. Across all treatment arms, MK-1084 demonstrated manageable safety and encouraging clinical activity, whether used alone or in combination with cetuximab—with or without chemotherapy.
The confirmed objective response rate (ORR) reached 38% with MK-1084 monotherapy and 46% when combined with cetuximab. In the triplet arm that added mFOLFOX6 chemotherapy, the overall confirmed ORR was also 38%. However, in patients receiving this triplet as a first-line treatment, the confirmed ORR rose to 58%, with an unconfirmed ORR of 92%, despite limited follow-up. These early signals suggest strong efficacy in the frontline setting.
The median duration of response was 10.8 months for the MK-1084 and cetuximab combination and had not yet been reached for the triplet regimen, indicating durable responses in some patients. Dose-limiting toxicities were infrequent, and overall safety remained manageable, even with combination therapies. Pharmacokinetic data also supported once-daily dosing of MK-1084, with a linear exposure pattern observed across the 25–200 mg range.
Altogether, these findings support continued investigation of MK-1084-based regimens—particularly in combination with cetuximab and chemotherapy—as a potential new standard for treating KRAS G12C–mutant colorectal cancer. Further data on progression-free and overall survival will provide more clarity as the study matures.
What People Are Saying About the KANDLELIT-001 Trial?
Dr. Jun Gong shared results from the KANDLELIT-001 Phase I dose-finding trial on his X page, underscoring the potential of the next-generation KRAS G12C inhibitor MK-1084 in advanced solid tumors, including metastatic colorectal cancer (mCRC). He posted:
“KANDLELIT-001 PhI dose-finding trial of next-gen #KRASG12C inh MK-1084 in #G12C mt adv solid tumors, for #mCRC cohort of pts (most w/1-2 prior lines) -> ORR 42-48% w/cetux & ORR 24-58% w/FOLFOX + cmab depending on # of prior lines). PFS & OS immature.”
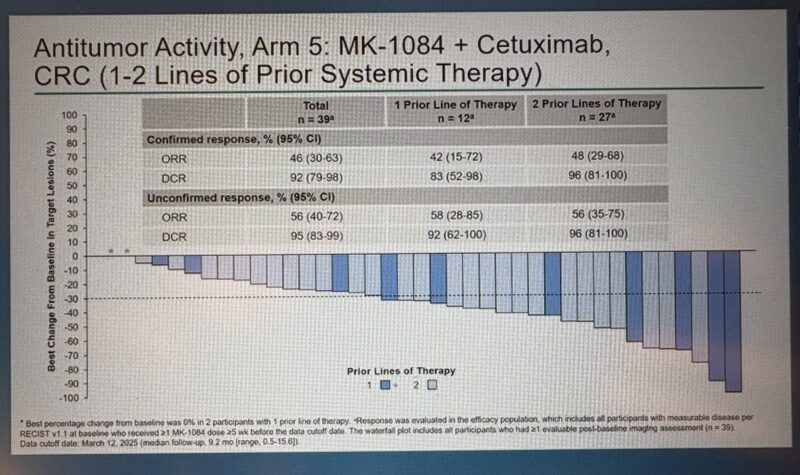
According to Dr. Gong, objective response rates (ORR) ranged from 42–48% when MK-1084 was combined with cetuximab, and 24–58% when combined with both FOLFOX and cetuximab, depending on the number of prior treatment lines. While progression-free survival (PFS) and overall survival (OS) data remain immature, these findings highlight the encouraging early activity of MK-1084-based combinations in KRAS G12C-mutant colorectal cancer.
More posts featuring ASCO25.