Muscle-invasive bladder cancer (MIBC) is a challenging disease with poor outcomes, particularly for patients who are ineligible for or refuse neoadjuvant chemotherapy (CT). The current standard of care—radical cystectomy (RC) combined with neoadjuvant CT—often faces limitations due to patient eligibility and tolerance. Survival outcomes for patients undergoing RC alone remain suboptimal, underscoring the urgent need for effective bladder-sparing therapies.
Immunotherapy with PD-1 inhibitors such as pembrolizumab (Pembro), as well as antibody-drug conjugates like sacituzumab govitecan (SG), have shown activity in MIBC in prior studies such as PURE-01 and SURE-01. Building on this, the phase 2 SURE-02 trial (NCT05535218) investigates the combination of neoadjuvant SG plus Pembro followed by adjuvant Pembro, aiming to maximize clinical complete response (cCR) rates and enable bladder preservation for patients with high-risk MIBC who cannot or choose not to undergo chemotherapy.
Study Design and Methods
SURE-02 enrolled patients aged 18 or older with histologically confirmed clinical stage T2 to T4N0M0 MIBC who were ineligible for or refused neoadjuvant CT and were scheduled for RC. Patients received four 21-day cycles of pembrolizumab 200 mg intravenously on Day 1 and sacituzumab govitecan 7.5 mg/kg on Days 1 and 8. After surgery, patients continued adjuvant pembrolizumab for up to 13 cycles.
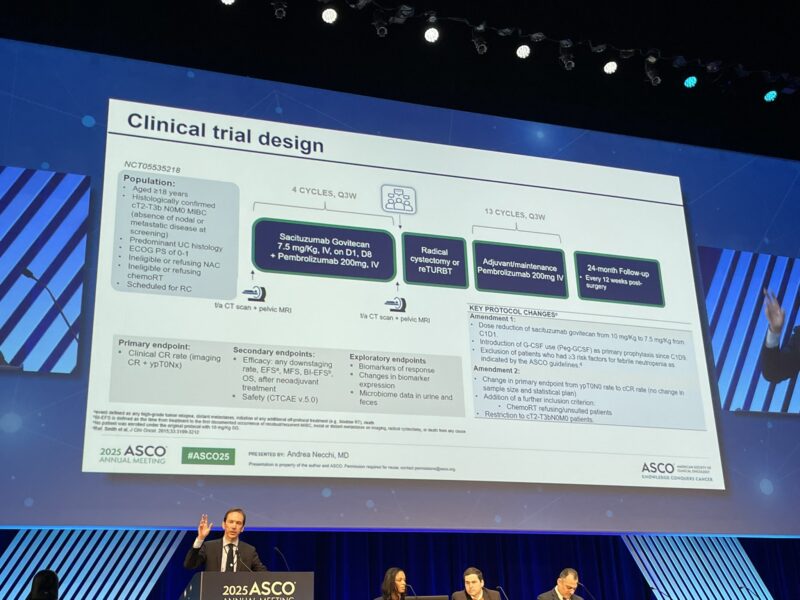
An innovative bladder-sparing approach was allowed for patients achieving a stringent clinical complete response (cCR), defined by a negative magnetic resonance imaging (MRI) scan and no residual tumor upon repeat transurethral resection of bladder tumor (reTURBT) with pathologic stage ypT0. The primary endpoint was the cCR rate, with success defined as achieving a cCR in ≥45% of patients, compared to a historical control of ≤30%. Secondary endpoints included pathologic response rates (ypT ≤1N0-x), safety profiles, and survival.
Additionally, the study incorporated comprehensive molecular analyses using the Decipher Bladder Genomic Subtyping Classifier (GSC) to evaluate transcriptome-wide tumor characteristics and correlate them with response.
Results: Encouraging Clinical and Molecular Findings
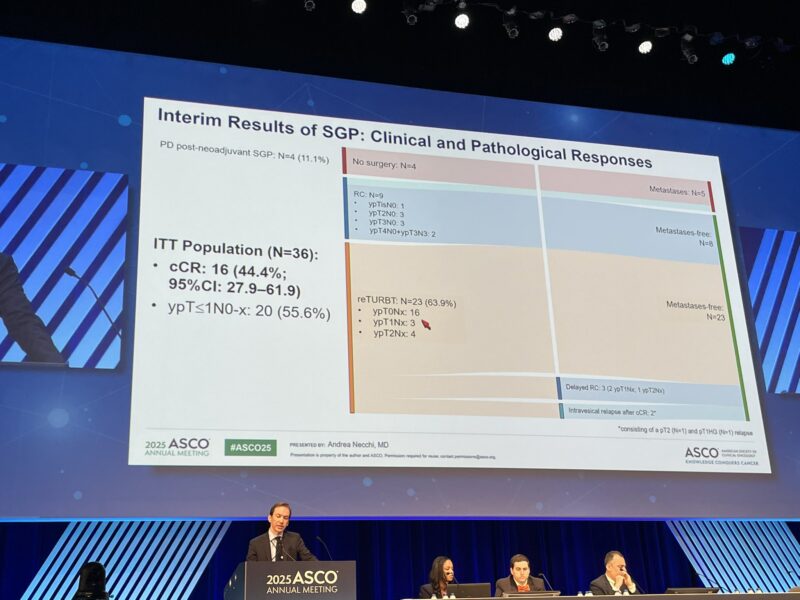
Between October 2023 and January 2025, 40 patients were treated, with 31 evaluable for efficacy. Among these, 64.5% presented with cT2 disease, and 38.7% had variant histologies confirmed centrally, reflecting a diverse patient population.
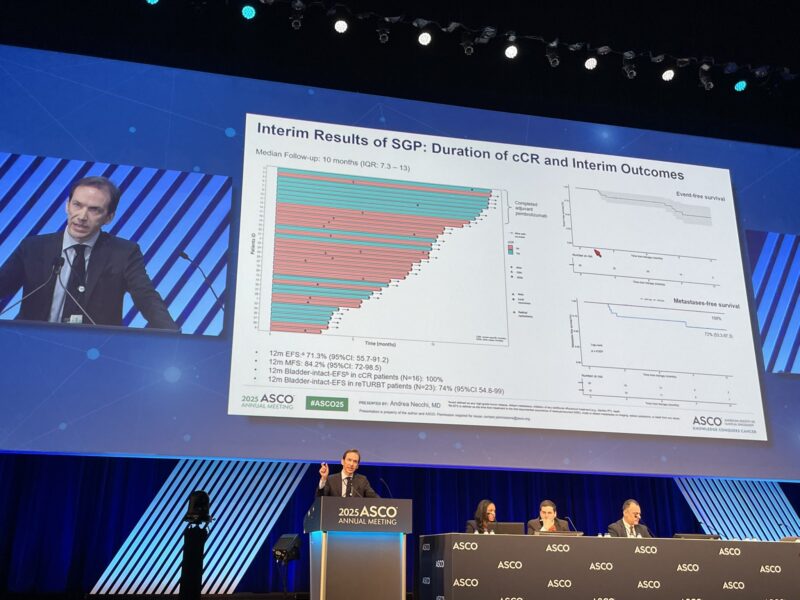
The interim analysis revealed a clinical complete response rate of 38.7% (12 patients; 95% CI: 21.8–57.8). All patients achieving cCR underwent reTURBT, confirming the absence of residual disease. The combined pathologic response rate—patients achieving ypT ≤1N0-x following RC or bladder preservation—was 51.6% (16 patients).
The treatment was generally well tolerated. Grade 3 or higher treatment-related adverse events (TRAEs) were observed in 12.9% of patients. Dose delays and omissions were minimal, and no dose reductions of sacituzumab govitecan were required. Importantly, these findings suggest a manageable safety profile, even with the combination therapy in the perioperative setting.
Molecular profiling offered valuable insights into tumor biology and response prediction. Transcriptome data from 23 patients revealed that luminal subtype tumors had significantly higher rates of complete pathologic response (73%) compared to non-luminal tumors (25%; p=0.04). According to Lund molecular subtypes, genomically unstable (GU) tumors showed a 67% ypT0 response rate, urothelial-like tumors 57%, basal/squamous 20%, and neuroendocrine-like tumors 0%.
Furthermore, tumors with a higher stromal signature (>median) were associated with non-ypT0 response (p=0.004), indicating that stromal features may influence resistance to therapy. Neither Trop2 nor TOP1 gene expression showed significant correlation with pathologic response, highlighting the complex molecular interplay governing therapeutic outcomes.
Clinical Implications and Future Directions
The interim results from SURE-02 provide compelling evidence that neoadjuvant sacituzumab govitecan combined with pembrolizumab offers meaningful clinical activity in patients with high-risk MIBC who cannot or opt not to receive chemotherapy. With a cCR rate approaching 40% and over half of patients achieving favorable pathologic outcomes, this combination demonstrates promise as a bladder-preserving strategy in this difficult-to-treat population.
The manageable safety profile further supports the feasibility of this regimen in the perioperative setting. Importantly, the incorporation of molecular biomarkers, particularly luminal subtype and genomic instability, may help personalize therapy, allowing clinicians to identify patients most likely to benefit from this bladder-sparing approach.
Completion of the full study accrual is ongoing, and these encouraging findings justify continued investigation of sacituzumab govitecan plus pembrolizumab in MIBC. Future analyses will provide data on long-term survival, durability of response, and quality of life outcomes.
What They’re Saying: Reactions toSURE-02 Trial at ASCO 2025
Zach Klaassen, Uro Onc from GACancerCenter, Assoc Prof, Resident PD, and Ronald W. Lewis, MD Chair of Uro Educ shared on X
SURE-02: Ph 2 neoadj SG + Pembro followed by resp-adapt bladder sparing and adj. Pembro in MIBC
n=36, Gr ≥3 TRAEs: 18.4%, Clinical CR rate: 44.4%, 12 mo EFS rate: 71.3%, 12 mo MFS rate: 84.2%, 12 mo bladder intact EFS in pts w/ clinical CR: 100%
SURE-02 trial of SG + pembro in MIBC, Included option for bladder preservation: 44% cCR, 100% metastasis free survival if cCR ERBB2 alt associated with response. MTAP loss with non-response
Key Takeaway Messages from the SURE-02 Phase 2 Trial
Trial Objective: The SURE-02 trial evaluated the combination of neoadjuvant sacituzumab govitecan (SG) plus pembrolizumab (Pembro), followed by adjuvant pembrolizumab, in patients with muscle-invasive bladder cancer (MIBC) who are ineligible or unwilling to receive chemotherapy.
Promising Complete Response: The trial reported a clinically meaningful clinical complete response (cCR) rate of 38.7%, allowing bladder preservation in nearly 40% of patients.
Overall Pathological Response: The rate of achieving a pathologic response of ypT≤1N0 was notable at 51.6%, highlighting significant anti-tumor efficacy of the SG + Pembro combination.
Manageable Safety Profile: Treatment-related severe adverse events (Grade ≥3) were relatively low (12.9%), demonstrating good tolerability and manageability of the combination therapy.
Molecular Predictors of Response: Biomarker analyses revealed differential response rates based on molecular subtypes:
- Higher ypT0 responses were observed in luminal compared to non-luminal tumors (73% vs. 25%).
- Lund genomically unstable (GU) subtype showed a notably high ypT0 response rate (67%).
Clinical Implication: The promising efficacy and manageable safety profile support continued accrual and investigation, positioning SG + Pembro as a potentially impactful perioperative strategy in MIBC, particularly for chemotherapy-ineligible patients.
Future Direction: These interim results emphasize the role of molecular characterization to personalize treatment strategies, potentially leading to more tailored bladder preservation protocols
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


