Immunotherapy for Skin Cancer: Types, Success Rate, Side Effects and More
Immunotherapy has become a powerful treatment option for skin cancer, especially for melanoma and certain non-melanoma skin cancers like Merkel cell carcinoma and advanced squamous cell carcinoma. By helping the immune system recognize and destroy cancer cells, it offers new hope for patients with advanced or treatment-resistant disease. This article will explore the types of immunotherapy used, their success rates, common side effects, and treatment costs, giving patients a clear understanding of what to expect.
What Is Immunotherapy for Skin Cancer?
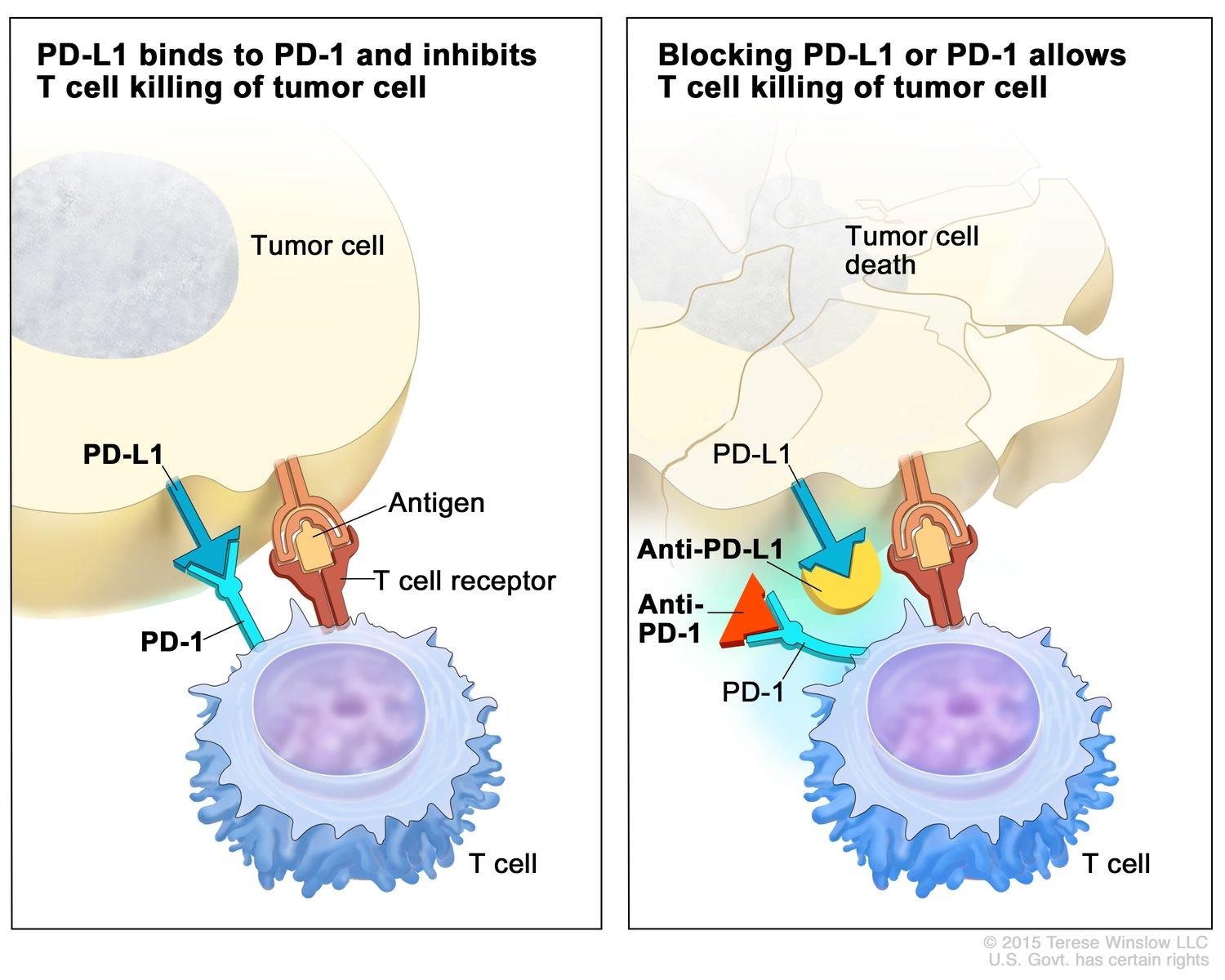
Immunotherapy is a form of cancer treatment that enhances the body’s immune system to recognize and attack cancer cells. Unlike traditional therapies that directly target the tumor, immunotherapy empowers the immune system to identify and eliminate malignant cells, offering the potential for durable responses and prolonged survival. A pivotal class of immunotherapy is immune checkpoint inhibitors, which target regulatory pathways in T cells to enhance anti-tumor responses. Under normal conditions, checkpoint proteins such as PD-1 (programmed death-1) act as brakes on the immune system, preventing overactivation that could damage healthy tissues. However, cancer cells can exploit these checkpoints to evade immune detection. By blocking PD-1 or its ligand PD-L1, checkpoint inhibitors release these brakes, enabling T cells to effectively attack tumors.
Pembrolizumab (Keytruda) and nivolumab (Opdivo) are prominent PD-1 inhibitors that have transformed the treatment landscape for various cancers, including skin malignancies. In melanoma, these agents have demonstrated significant efficacy. For instance, a study published in The Guardian reported that a combination of nivolumab and ipilimumab led to a 10-year survival rate of 52% in patients with advanced melanoma, a substantial improvement over previous outcomes. In the context of cutaneous squamous cell carcinoma (cSCC), pembrolizumab has shown promise. A study published in Frontiers in Oncology evaluated the effectiveness of pembrolizumab in patients with advanced cSCC and found an overall response rate of 46.1%, indicating its potential as a therapeutic option for this patient population.
These findings underscore the transformative impact of checkpoint inhibitors like pembrolizumab and nivolumab in the management of skin cancers, offering patients improved survival outcomes and a new avenue for treatment.
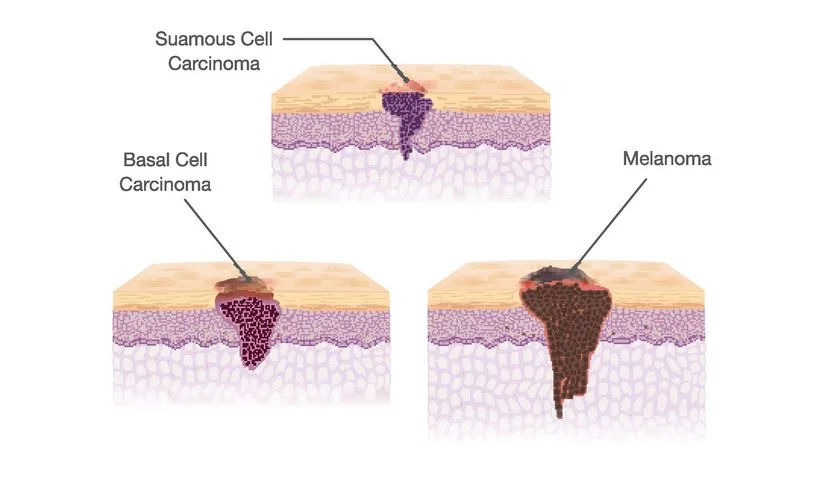
What Are the Types of Immunotherapy for Skin Cancer?
Immunotherapy for skin cancer includes several treatment types that work by boosting the body’s natural defenses to fight cancer. The main types used across melanoma, squamous cell carcinoma (SCC), and basal cell carcinoma (BCC)include:
-
Immune checkpoint inhibitors (e.g., pembrolizumab, nivolumab)
-
Oncolytic virus therapy (e.g., talimogene laherparepvec, or T-VEC)
-
Topical immunotherapy creams (e.g., imiquimod)
Each of these therapies plays a role depending on the type and stage of the skin cancer. In the following sections, we will explore how they are used, their effectiveness, and what patients can expect.
Checkpoint Inhibitors
Checkpoint inhibitors, such as pembrolizumab (Keytruda) and nivolumab (Opdivo), have transformed the treatment landscape for various cancers, including melanoma and advanced cutaneous squamous cell carcinoma (cSCC). These therapies function by targeting the programmed death-1 (PD-1) receptor, a critical immune checkpoint that, when engaged by its ligands (PD-L1 or PD-L2), downregulates T-cell activity, allowing cancer cells to evade immune surveillance. By inhibiting PD-1, these agents enhance T-cell responses against tumor cells.
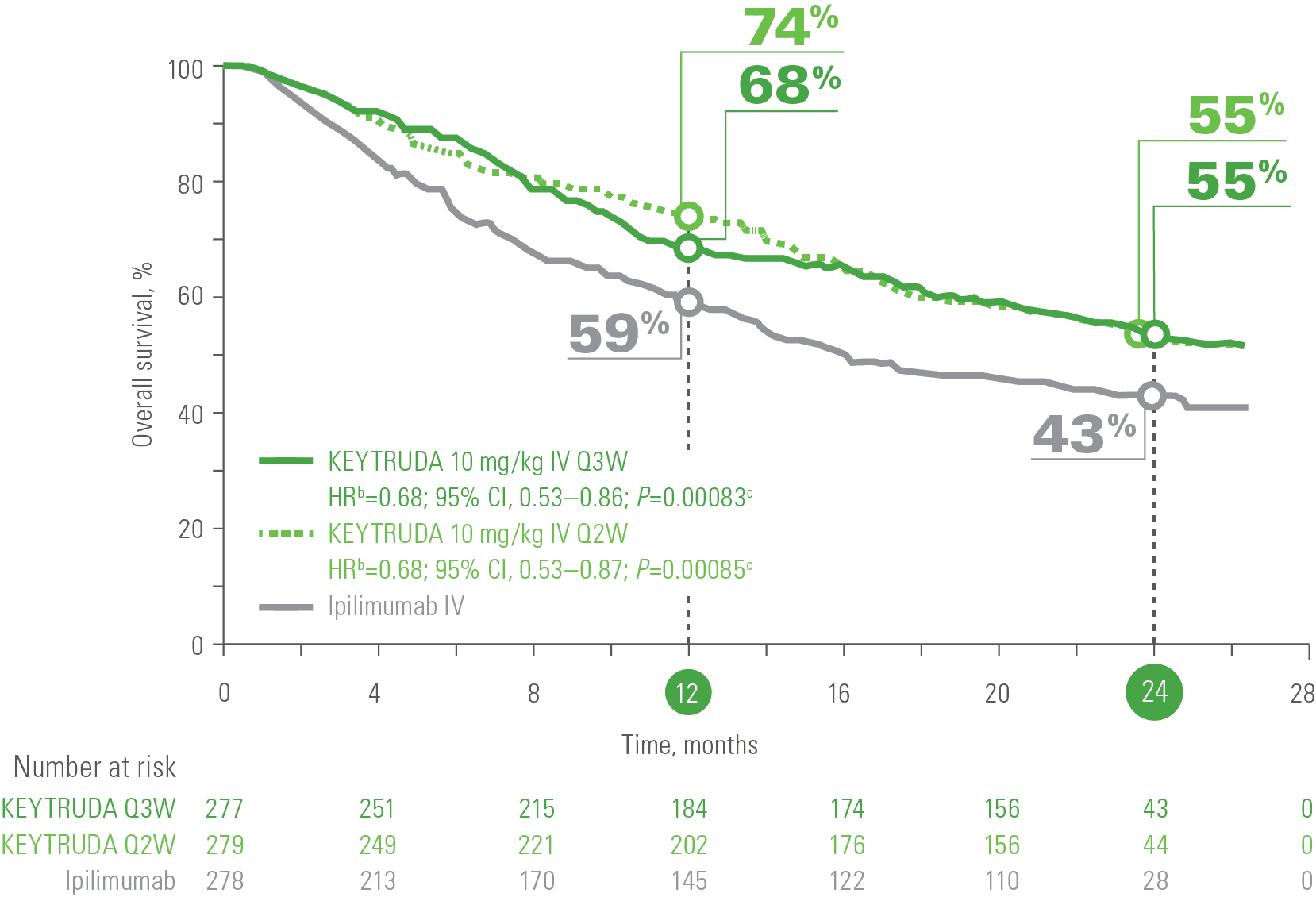
In melanoma, the efficacy of pembrolizumab was demonstrated in the KEYNOTE-006 trial, a phase III study led by Antoni Ribas and colleagues, published in the New England Journal of Medicine in 2015. The study compared pembrolizumab to ipilimumab in patients with advanced melanoma and found that pembrolizumab significantly improved progression-free survival (PFS) and overall survival (OS). Specifically, the 6-month PFS was 47.3% with pembrolizumab versus 26.5% with ipilimumab, and the 12-month OS was 74.1% versus 58.2%, respectively.
For advanced cSCC, the efficacy of pembrolizumab was evaluated in a study by Rischin et al., published in The Lancet Oncology in 2020. This multicenter, phase II trial assessed pembrolizumab in patients with unresectable or metastatic cSCC. The results demonstrated an objective response rate (ORR) of 50%, with 17% achieving complete responses and 33% partial responses. The median duration of response was not reached, indicating sustained efficacy in responders.
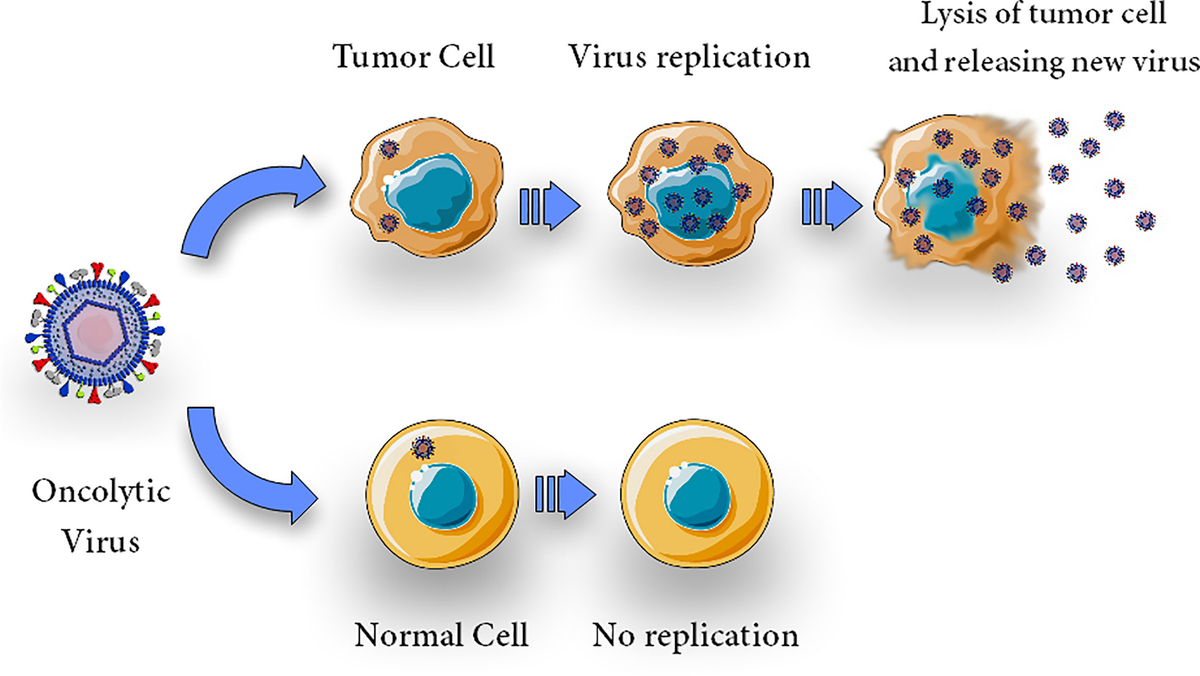
Oncolytic Virus Therapy
Oncolytic virus therapy represents a novel approach in cancer treatment, utilizing genetically engineered viruses to selectively infect and destroy cancer cells while sparing normal tissues. Talimogene laherparepvec (T-VEC), a modified herpes simplex virus type 1, exemplifies this strategy and has been approved for the treatment of advanced melanoma.
T-VEC is engineered to replicate within tumor cells, leading to cell lysis and the release of tumor-derived antigens.Concurrently, it expresses granulocyte-macrophage colony-stimulating factor (GM-CSF), which recruits and matures dendritic cells, thereby enhancing the systemic anti-tumor immune response. This dual mechanism not only targets the injected tumor but may also elicit responses in distant metastases.
The efficacy of T-VEC was demonstrated in the OPTiM trial, a randomized, open-label, phase III study involving patients with unresectable stage IIIB-IV melanoma. Conducted by Robert H. I. Andtbacka and colleagues, the trial compared intralesional T-VEC to subcutaneous GM-CSF. Results indicated a durable response rate (DRR) of 16.3% in the T-VEC group versus 2.1% in the GM-CSF group. The overall response rate (ORR) was 26.4% for T-VEC compared to 5.7% for GM-CSF. Notably, patients with earlier-stage disease (stage IIIB-IVM1a) exhibited a more pronounced benefit, with a DRR of 33% and an ORR of 40.5%. These findings underscore T-VEC’s potential, particularly in patients with less advanced metastatic melanoma.
Subsequent analyses have provided further insights into T-VEC’s role in melanoma treatment. A final 5-year follow-up of a phase III trial evaluating neoadjuvant T-VEC plus surgery versus surgery alone reported a 2-year recurrence-free survival (RFS) of 29.5% for the combination therapy compared to 16.5% for surgery alone. This benefit persisted at the 3-year mark, suggesting a sustained advantage with the addition of T-VEC. Real-world data have also corroborated T-VEC’s efficacy and safety profile. A study assessing T-VEC in routine clinical practice found it to be well-tolerated and effective in patients with unresectable, locoregional, and injectable metastatic melanoma.
Immunotherapy Creams
Topical immunotherapy, particularly with agents like imiquimod, has emerged as a non-invasive treatment option for certain early-stage non-melanoma skin cancers, notably superficial basal cell carcinoma (sBCC) and actinic keratosis (AK). Imiquimod is a synthetic imidazoquinoline that functions as an immune response modifier, enhancing the body’s innate and adaptive immune mechanisms to target and eliminate neoplastic cells. The mechanism of action of imiquimod involves binding to Toll-like receptors (TLR) 7 and 8 on dendritic cells and macrophages, leading to the activation of nuclear factor kappa-B (NF-κB). This activation results in the production of proinflammatory cytokines such as interferon-alpha (IFN-α), tumor necrosis factor-alpha (TNF-α), and various interleukins. These cytokines stimulate a Th1-mediated immune response, promoting the recruitment and activation of T cells that recognize and destroy malignant cells.
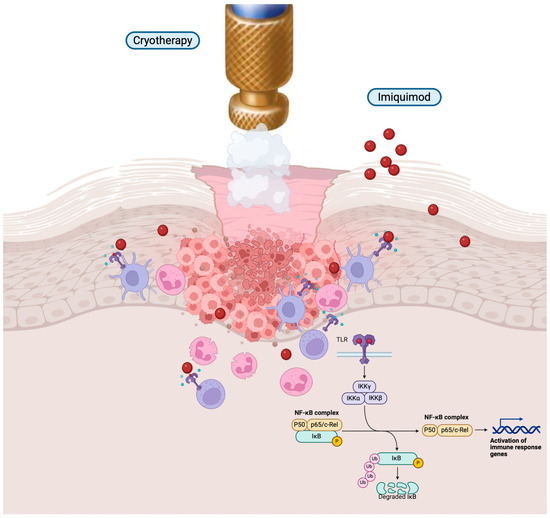
In the treatment of superficial basal cell carcinoma, imiquimod has demonstrated efficacy. A systematic review by Bath-Hextall et al., published in the Cochrane Database of Systematic Reviews in 2014, analyzed data from multiple randomized controlled trials. The review reported that imiquimod achieved histological clearance rates ranging from 70% to 100%, depending on the study parameters and treatment regimens. The authors concluded that imiquimod is an effective treatment for sBCC, particularly for patients who are not candidates for surgical interventions. Regarding actinic keratosis, imiquimod has been shown to be effective in clearing lesions and reducing the risk of progression to squamous cell carcinoma. A study by Jansen et al., published in the Journal of the American Academy of Dermatology in 2009, evaluated the long-term efficacy of imiquimod in patients with multiple AK lesions. The study found that 88% of patients achieved complete clearance of lesions after a 12-week treatment period, with sustained results observed at a 12-month follow-up.
The application protocol for imiquimod typically involves applying the 5% cream to the affected area once daily, five times per week for six weeks, ensuring the cream remains on the skin for approximately eight hours before washing off.Common local skin reactions include erythema, edema, and erosion, which are generally indicative of an active immune response and correlate with treatment efficacy. Systemic side effects are rare but may include flu-like symptoms.
How Effective Is Immunotherapy for Skin Cancer?
Immunotherapy has significantly advanced the treatment of skin cancers, particularly melanoma, cutaneous squamous cell carcinoma (cSCC), and basal cell carcinoma (BCC). By harnessing the body’s immune system, these therapies have improved response rates and survival outcomes.
- Melanoma: The CheckMate 067 trial evaluated the efficacy of nivolumab combined with ipilimumab versus monotherapy in patients with advanced melanoma. At a 10-year follow-up, the overall survival rates were 43% for the combination therapy, 37% for nivolumab alone, and 19% for ipilimumab alone. The median overall survival was 71.9 months with the combination, 36.9 months with nivolumab, and 19.9 months with ipilimumab.
- Cutaneous Squamous Cell Carcinoma (cSCC): The KEYNOTE-629 study assessed pembrolizumab in patients with locally advanced or recurrent/metastatic cSCC. The objective response rates were 50% for locally advanced cases and 35.2% for recurrent/metastatic cases. These responses were durable, with a median duration not reached during the study period. Additionally, the FDA approved a PD-L1 antibody, Unloxcyt, for cSCC based on a study involving 109 patients, showing an overall response rate of 47% in metastatic cases and 48% in locally advanced cases.
- Basal Cell Carcinoma (BCC): For advanced BCC, the PD-1 inhibitor cemiplimab demonstrated an objective response rate of 31% in a pivotal phase II trial involving patients who were unsuitable for or had recurrence after Hedgehog inhibitor treatment. The median time to response was 4.3 months, and the disease control rate was 60%.
What Are the Side Effects of Immunotherapy for Skin Cancer?
Immunotherapy for Skin Cancer can result in a range of side effects that vary in severity. While some side effects are common and generally manageable, others can be serious and require close monitoring.
Common Side Effects
While immunotherapy has transformed treatment outcomes for skin cancer, it can also lead to side effects due to its activation of the immune system. Unlike chemotherapy, which attacks rapidly dividing cells, immunotherapy stimulates immune responses that may mistakenly target healthy tissues, leading to what are known as immune-related adverse events (irAEs). Among the most common side effects are fatigue, skin reactions, and digestive issues, each of which can affect a patient’s quality of life in different ways.
- Fatigue is one of the most frequently reported side effects and can range from mild tiredness to severe exhaustion. According to the American Cancer Society, up to 40–80% of patients receiving immunotherapy experience fatigue during or after treatment. It can persist for weeks or even months after therapy ends and significantly interfere with daily activities, concentration, and sleep quality. Management strategies include light physical activity (e.g., walking or stretching), good sleep hygiene, proper hydration, and addressing contributing factors like anemia or thyroid dysfunction.
- Skin-related side effects such as rashes, itching, or dryness are also common, especially with checkpoint inhibitors. These reactions are often mild but can progress to more severe forms such as dermatitis or vitiligo in melanoma patients. The skin changes can affect appearance and self-esteem, sometimes leading to social discomfort.
Treatment typically involves topical corticosteroids, antihistamines for itching, and moisturizers. In moderate to severe cases, systemic steroids may be required, and dermatologic consultation is recommended. - Gastrointestinal side effects, including diarrhea, nausea, abdominal pain, and in more serious cases, colitis, occur when immune activation affects the lining of the digestive tract. These symptoms can lead to weight loss, dehydration, and electrolyte imbalances, particularly in older patients or those with pre-existing GI conditions.
Mild cases can be managed with dietary adjustments and anti-diarrheal medications. However, persistent or severe symptoms often require corticosteroids or immunosuppressive therapy (such as infliximab). Early detection is crucial to prevent complications like bowel perforation.

Serious Side Effects
While immunotherapy has brought transformative outcomes in cancer treatment, it can also lead to serious immune-related adverse events (irAEs). Among the most concerning of these are pneumonitis and colitis—conditions where the immune system, activated by treatment, mistakenly targets healthy lung or bowel tissue. These side effects, although less common than fatigue or skin rash, can be severe and even life-threatening if not recognized and treated early.
Immune-related pneumonitis involves inflammation of the lung tissue and may present with shortness of breath, dry cough, and low-grade fever. The symptoms often resemble infections or worsening lung disease, making diagnosis challenging. In a case reported by Andric et al. (2023) in Case Reports in Oncology, a 68-year-old lung cancer patient developed lethal pneumonitis after treatment with the checkpoint inhibitor durvalumab. Despite early discontinuation of the drug and initiation of high-dose corticosteroids, the patient’s respiratory condition deteriorated rapidly. This case underscores the importance of close respiratory monitoring, especially in patients with pre-existing lung conditions.
When pneumonitis is suspected, oncologists typically pause immunotherapy immediately and start high-dose corticosteroids, such as prednisone. In more severe or steroid-resistant cases, additional immunosuppressive medications like infliximab or mycophenolate mofetil may be required. Coordination with pulmonologists is critical, and follow-up imaging often guides decisions about resuming or permanently discontinuing immunotherapy. Another potentially serious irAE is immune-mediated colitis, which commonly presents with frequent diarrhea, abdominal pain, and, in severe cases, blood in the stool. Left untreated, it can lead to dehydration, colon inflammation, or even perforation. In a case reported by Tang et al. (2020) in Cureus, a patient undergoing immunotherapy for melanoma developed severe diarrhea unresponsive to corticosteroids. The patient was later treated successfully with infliximab, a TNF-alpha inhibitor, which helped resolve the symptoms.
For oncologists, early grading of colitis symptoms is key. Mild cases can often be managed with diet changes and anti-diarrheal medication, but moderate to severe cases require immunosuppressive therapy. Colonoscopy may be needed to rule out infections and confirm diagnosis, and GI specialists are often brought into the care team. In both pneumonitis and colitis, patient education is essential. Oncologists stress the importance of reporting new or unusual symptoms immediately—even weeks or months after starting immunotherapy. Many irAEs can appear late into treatment or even after it ends.
Though serious, these side effects are manageable with early intervention, and most patients can resume treatment after recovery, depending on severity. With multidisciplinary support and close monitoring, oncologists can safely navigate these risks while maintaining the life-saving potential of immunotherapy.
Immunotherapy for Advanced Skin Cancer
Advanced skin cancers, such as metastatic melanoma and cutaneous squamous cell carcinoma (cSCC), have historically posed significant treatment challenges. Recent advancements in immunotherapy have markedly improved patient outcomes, with agents like pembrolizumab (Keytruda) playing a pivotal role in metastatic melanoma management, and combination therapies showing promise in cSCC treatment.
- Pembrolizumab in Metastatic Melanoma: Pembrolizumab, a PD-1 inhibitor, has demonstrated substantial efficacy in treating metastatic melanoma. Long-term data from clinical trials indicate a 10-year overall survival rate of 34.0% for patients treated with pembrolizumab, compared to 23.6% for those receiving ipilimumab, highlighting a significant survival benefit. The median overall survival was notably longer with pembrolizumab at 32.7 months, versus 15.9 months with ipilimumab. These findings underscore pembrolizumab’s role in extending survival for patients with advanced melanoma.
- Combination Therapies in Cutaneous Squamous Cell Carcinoma: For advanced cSCC, combination immunotherapies have shown promise. Checkpoint inhibitors like cemiplimab and pembrolizumab have achieved high response rates and improved survival outcomes with manageable toxicity profiles.These agents have become integral in the systemic treatment of cSCC, offering effective options for patients with advanced disease.
Additionally, the recent FDA approval of Unloxcyt, a PD-L1 antibody, for the treatment of cSCC, was based on a study involving 109 patients, showing an overall response rate of 47% in metastatic cases and 48% in locally advanced cases.
How Is Immunotherapy Administered?
Immunotherapy administration varies depending on the type of treatment and cancer. The most common method is intravenous (IV) infusion, typically given in a clinic or hospital. Drugs like pembrolizumab and nivolumab are usually infused over 30 to 60 minutes, with treatments scheduled every 2 to 6 weeks, depending on the specific regimen.
For early-stage non-melanoma skin cancers, topical immunotherapy such as imiquimod cream is applied directly to the skin at home. This is usually done once daily, 5 times a week, for 6 to 12 weeks, depending on the lesion and response. Treatment duration can range from a few months to over a year, and patients are monitored regularly for both effectiveness and side effects.
What Should Patients Expect During Treatment?
The patient experience during immunotherapy begins with a thorough consultation where the oncologist explains the treatment plan, goals, and potential side effects. Before starting, baseline tests are done (blood work, imaging), and patients receive education on what to expect. During treatment, IV infusions are typically painless and take 30–60 minutes. Patients may rest, read, or listen to music during sessions. Nurses monitor vital signs and comfort throughout.
Side effects such as fatigue, skin rash, or mild digestive issues may occur. These are managed with medication, hydration, rest, or dietary adjustments. More serious effects are monitored closely with routine checkups. Follow-up care includes regular lab tests and scans to track response and catch any delayed side effects. Open communication with the care team is key to managing treatment smoothly and effectively.
How Long Does It Take to See Results from Immunotherapy?
The timeline for seeing results from immunotherapy varies depending on the type of cancer, the drug used, and the individual’s immune response. Unlike chemotherapy, which may produce rapid changes, immunotherapy responses can take weeks to months to become noticeable.
According to clinical trial data, partial or complete responses to checkpoint inhibitors like pembrolizumab or nivolumab in melanoma and squamous cell carcinoma often begin to appear within 6 to 12 weeks, though some patients may experience delayed responses beyond 3–6 months. In a pooled analysis of immunotherapy trials for melanoma, the median time to response was 2.8 months, but some patients responded as late as 12 months after treatment initiation (Hodi et al., Journal of Clinical Oncology, 2016). Importantly, some tumors may initially appear to grow—a phenomenon called pseudoprogression—before shrinking, due to immune cell infiltration. This is why continued monitoring with scans and lab tests is critical during the first few months of therapy.
What Are the Costs of Immunotherapy for Skin Cancer?
Immunotherapy for skin cancer, particularly treatments like pembrolizumab (Keytruda) and nivolumab (Opdivo), has revolutionized patient outcomes but comes with significant costs. For instance, the list price for each indicated dose of Keytruda, administered every six weeks, is $23,590.88, though actual out-of-pocket expenses vary based on insurance coverage.
Combination therapies, such as ipilimumab (Yervoy) combined with nivolumab, further increase expenses. A study analyzing the costs associated with this combination in a real-world setting found that while effective, the financial impact on the healthcare system is substantial. Over time, the financial burden of treating advanced melanoma has escalated. Between 2007 and 2012, patients incurred an average cost of $47,739 for stage IV treatment. This figure surged to $117,450 for patients treated between 2018 and 2019, reflecting the introduction and adoption of newer, more expensive therapies.
Given these substantial costs, patients are encouraged to explore financial assistance programs offered by pharmaceutical companies and non-profit organizations to help mitigate out-of-pocket expenses.
How Does Immunotherapy Compare to Other Treatments for Skin Cancer?
In the treatment of advanced-stage skin cancer, including melanoma and squamous cell carcinoma, various modalities such as surgery, chemotherapy, targeted therapy, and immunotherapy are employed. Each approach has distinct mechanisms, efficacy profiles, and side effect considerations.
- Surgery is often the first-line treatment for localized skin cancers, aiming to remove the tumor entirely. However, in advanced stages where metastasis has occurred, surgery alone may be insufficient.
- Chemotherapy utilizes cytotoxic drugs to kill rapidly dividing cancer cells. While effective, it often affects healthy dividing cells, leading to side effects such as hair loss, nausea, and increased infection risk. According to the American Cancer Society, chemotherapy can cause significant side effects, including fatigue and increased susceptibility to infections.
- Targeted therapy focuses on specific genetic mutations within cancer cells. For instance, BRAF inhibitors are used in melanomas with BRAF mutations. These therapies can be effective but may lead to side effects like skin rashes. The American Cancer Society notes that targeted drugs can cause rashes, often appearing on the scalp, face, neck, chest, and upper back.
- Immunotherapy enhances the body’s immune system to recognize and attack cancer cells. Agents such as pembrolizumab and nivolumab have shown significant efficacy in advanced melanoma. A study published in Cancer Immunology, Immunotherapy by F. Stephen Hodi et al. reported that immunotherapy has improved survival rates in patients with stage IV melanoma, with benefits persisting even after treatment cessation. In terms of side effects, immunotherapy is generally associated with fewer acute adverse events compared to chemotherapy. Common side effects include fatigue, skin rash, and mild gastrointestinal symptoms. However, as noted by the American Cancer Society, immunotherapy can still pose significant risks, and doctors employ strategies to mitigate immune-related side effects. Furthermore, immunotherapy has demonstrated the potential for durable responses. A clinical trial reported by The Guardian found that over half of patients with advanced melanoma treated with a combination of ipilimumab and nivolumab survived for at least 10 years, suggesting the possibility of a cure in some cases.
What Research Is Being Done on Immunotherapy for Skin Cancer?
Recent advancements in immunotherapy have significantly improved outcomes for patients with advanced skin cancers, particularly melanoma. Ongoing clinical trials are exploring novel drugs and combination therapies to enhance efficacy and extend survival rates.
A landmark study published in The New England Journal of Medicine by Larkin et al. reported that the combination of nivolumab and ipilimumab resulted in a 10-year survival rate of 52% for patients with advanced melanoma. This combination therapy demonstrated a continued, ongoing survival benefit compared to monotherapy. Innovative treatments are also under investigation. A phase II trial examined the combination of tocilizumab, an IL-6 receptor inhibitor, with ipilimumab and nivolumab in advanced melanoma patients. The results indicated an objective response rate of 70% at the 6-month follow-up, with a grade 3/4 immune-related adverse event rate of 25%.
Additionally, the mRNA-4157/V940 vaccine, developed by Moderna, has shown promise. In combination with pembrolizumab, this personalized mRNA cancer vaccine reduced the risk of cancer recurrence by 44% in resected stage IIIB-IV melanoma patients. The vaccine has received Breakthrough Therapy Designation from the FDA. Neoadjuvant therapies are being explored to improve surgical outcomes. A study involving vidutolimod, a novel agent, combined with nivolumab as pre-surgical treatment for stage III cutaneous melanoma, achieved tumor control in 55% of patients.
In the adjuvant setting, pembrolizumab has demonstrated efficacy. A phase III study showed that pembrolizumab improved the 1-year recurrence-free survival rate to 75.4% in patients with resected stage III melanoma, compared to 61.0% with placebo.
How Can Patients Support Their Immune System During Treatment?
Supporting the immune system during immunotherapy is essential for patients undergoing treatment for skin cancer. While the therapy itself boosts immune activity, adopting healthy lifestyle habits can further enhance the body’s ability to respond.
Patients are encouraged to eat a balanced, nutrient-rich diet that includes fruits, vegetables, lean proteins, and whole grains. Studies have shown that antioxidants, fiber, and healthy fats support immune cell function during cancer treatment. Staying hydrated is equally important—water helps maintain circulation and supports detoxification, which is critical during immunotherapy. Regular, gentle physical activity, such as walking or yoga, has been shown to improve immune regulation and reduce fatigue. According to the American Cancer Society, exercise during treatment can also improve mood and help manage side effects. Stress reduction is another key factor. Chronic stress can weaken immune responses, so practices like meditation, deep breathing, or counseling are highly recommended. Finally, patients should take care to avoid infections by practicing good hygiene, avoiding sick contacts, and staying up to date on vaccines when appropriate.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is immunotherapy for skin cancer?
Immunotherapy is a cancer treatment that helps the immune system detect and destroy skin cancer cells, especially in advanced or resistant cases.
Which skin cancers are treated with immunotherapy?
Immunotherapy is commonly used for melanoma, Merkel cell carcinoma, advanced squamous cell carcinoma, and some basal cell carcinomas.
What are immune checkpoint inhibitors?
They are drugs like pembrolizumab (Keytruda) and nivolumab (Opdivo) that block PD-1 or PD-L1 proteins, boosting the immune response against cancer.
Is there an immunotherapy cream for skin cancer?
Yes, imiquimod is a topical cream used for early-stage non-melanoma skin cancers like superficial basal cell carcinoma and actinic keratosis.
How effective is immunotherapy for melanoma?
Studies show long-term survival benefits, with some patients living more than 10 years after treatment with checkpoint inhibitors.
What are the common side effects of immunotherapy?
Fatigue, skin rashes, and digestive issues like diarrhea are common; most are manageable with supportive care.
Can immunotherapy cause serious side effects?
Yes, serious side effects include pneumonitis and colitis. These require prompt treatment with steroids or immunosuppressants.
How is immunotherapy given to skin cancer patients?
It’s usually given by IV infusion every 2 to 6 weeks. For some early skin cancers, a cream like imiquimod is applied directly to the skin.
How long does it take for immunotherapy to work?
Responses may appear within 6–12 weeks, but some patients respond later. Regular scans track progress.
Is immunotherapy for skin cancer expensive?
Yes, treatments like Keytruda can cost over $20,000 per dose. Insurance and assistance programs may help cover costs.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
