ADCs (antibody-drug conjugates) were the subject of a key presentation by Dr. Miranda Chen, United States at MASCC 2025, highlighting new real-world data on their hematologic toxicity profiles. In a large, multi-center analysis across five University of California hospitals, researchers evaluated the incidence and predictors of grade ≥3 neutropenia in patients receiving commonly used ADCs. With over 1,500 patients included, this study offers important insights into both drug-specific and patient-related risk factors, informing clinical strategies for safer, more personalized use of ADCs in oncology.

Background
Antibody-drug conjugates (ADCs) are a class of targeted cancer therapies that combine the specificity of monoclonal antibodies with the cytotoxic potency of chemotherapy. By linking a cytotoxic drug (the “payload”) to an antibody that targets tumor-specific antigens, ADCs aim to deliver the therapeutic agent directly to cancer cells while sparing healthy tissue. This mechanism allows for more precise tumor targeting and reduced systemic toxicity compared to traditional chemotherapy.
Over the past decade, ADCs have gained regulatory approval across a range of malignancies, including breast, bladder, hematologic, and gynecologic cancers. However, despite their targeted approach, many ADCs are associated with off-target effects, most notably hematologic toxicities such as neutropenia. Understanding which patients are most at risk for these adverse effects is crucial for optimizing safety, maintaining dose intensity, and improving treatment outcomes.
Study Design and Methodology
This retrospective observational study utilized data from the University of California Health Data Warehouse, focusing on patients who received at least one dose of any of the ten most commonly used ADCs between November 2017 and June 2024. The ADCs included:
- Fam-trastuzumab deruxtecan
- Ado-trastuzumab emtansine
- Brentuximab vedotin
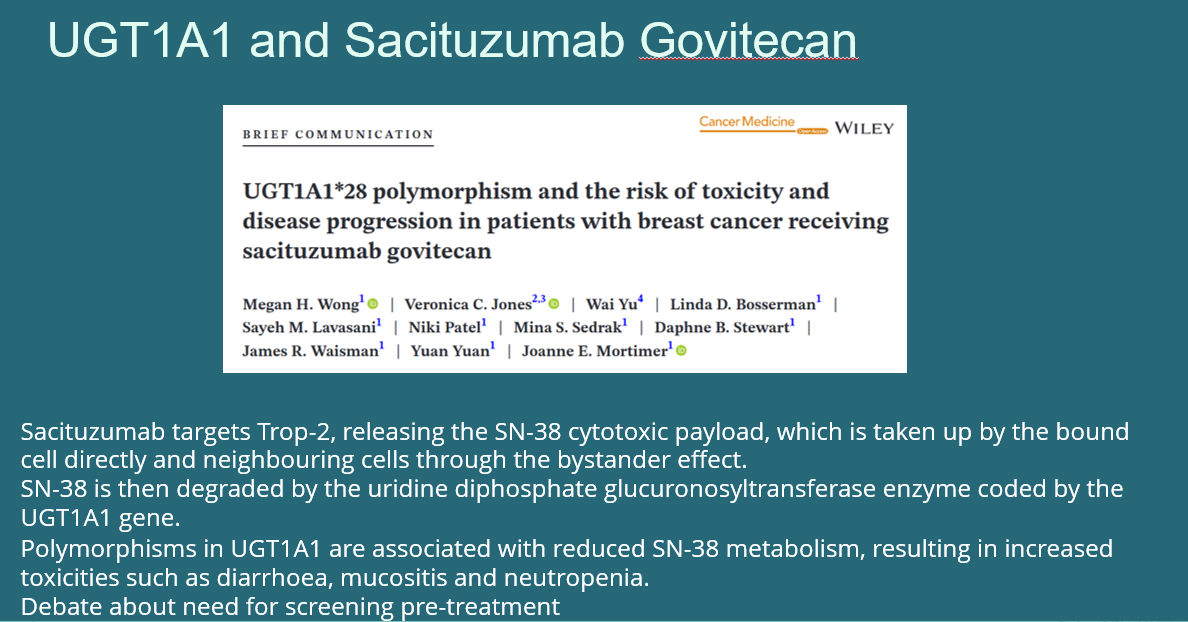
- Sacituzumab govitecan
- Enfortumab vedotin
- Gemtuzumab ozogamicin
- Inotuzumab ozogamicin
- Polatuzumab vedotin
- Belantamab mafodotin
- Bisotumab vedotin
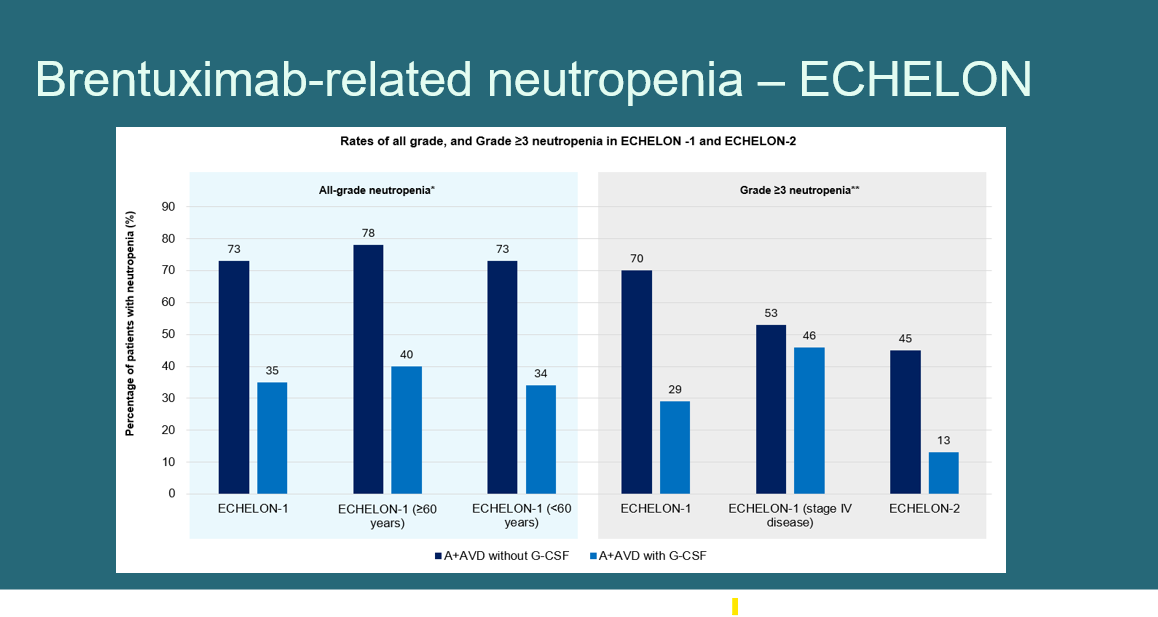
The primary outcomes were:
- Grade ≥3 neutropenia following the first ADC dose
- Hospitalization due to treatment-related complications
Patient demographics, malignancy types, comorbidities, and baseline lab abnormalities were extracted and analyzed. Statistical significance was determined through chi-square testing and multivariate logistic regression, incorporating both clinically relevant and statistically significant variables from univariate analysis.
Key Findings
Out of 1,527 patients
- Gemtuzumab ozogamicin was most strongly associated with high-grade neutropenia (OR=60.50, 95% CI: 9.89–376.00, p<0.01)
- Inotuzumab ozogamicin also posed a significant risk (OR=16.90, 95% CI: 2.87–101.00, p<0.01)
- Fam-trastuzumab deruxtecan and ado-trastuzumab emtansine were linked to significantly lower risk of neutropenia (OR=0.31 and OR=0.01 respectively)
In addition to the drug type, the following patient-related factors were independently associated with increased risk of severe neutropenia:
- Pre-existing anemia (OR=6.29)
- Baseline liver dysfunction (OR=2.38)
- Immunodeficiency disorders (OR=1.92)
- Breast malignancy (OR=3.68)
Notably, no variables were significantly associated with hospitalization, suggesting that while neutropenia is a common and measurable toxicity, it does not necessarily translate to hospital-level complications in this population—though supportive care protocols may have mitigated that risk.
Clinical Implications
This MASCC 2025 study highlights the heterogeneity of toxicity risk across ADC agents, reinforcing the importance of understanding both drug-specific and host-related factors when initiating ADC therapy. For agents like gemtuzumab and inotuzumab, which carry higher hematologic risk, closer monitoring, prophylactic growth factor use, or treatment modification may be warranted in high-risk patients.
Furthermore, the identification of modifiable or manageable risk factors—such as pre-treatment anemia and liver dysfunction—opens avenues for prehabilitation strategies to improve patient resilience prior to ADC initiation. This aligns with broader efforts in precision supportive care in oncology.
Conclusion and Future Directions
The findings presented by Chen et al. at MASCC 2025 provide important real-world evidence on ADC-associated neutropenia, helping to inform clinical decision-making and risk communication. As the use of ADCs expands across cancer types, these results emphasize the need for predictive models that integrate clinical data, drug pharmacology, and patient comorbidities.
Future prospective studies may explore:
- Integration of biomarkers (e.g., pharmacogenomic or immune profiling)
- Impact of dose modification strategies or prophylactic interventions
- Real-world outcomes such as treatment delays, dose reductions, or infection-related events
- Ultimately, optimizing the safety of ADCs through personalized risk asses
You can read the full article here.


