Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a major cause of cancer-related mortality worldwide. Although the combination of atezolizumab plus bevacizumab (A+B) has become the standard first-line treatment for unresectable HCC, many patients eventually experience disease progression, and the optimal second-line therapy remains uncertain.
This article explores the LEVIATHAN study, a large, multinational observational analysis designed to compare lenvatinib and sorafenib as second-line treatment options following A+B failure. By leveraging real-world data and propensity score matching, the study provides important insights into treatment sequencing and survival outcomes in advanced HCC.
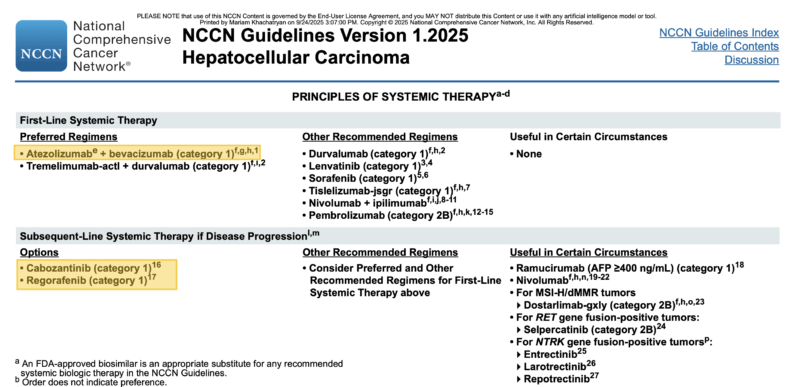
2025 Guideline: What is standard treatment for HCC?
According to NCCN Guidelines v1.2025, the preferred first-line systemic regimens for unresectable or advanced HCC are:
- Atezolizumab + bevacizumab
- Tremelimumab-actl + durvalumab (STRIDE regimen)
Other recommended regimens include durvalumab monotherapy, lenvatinib, sorafenib, tislelizumab, nivolumab + ipilimumab, and pembrolizumab (category 2B).
For subsequent-line therapy after progression, NCCN recommends cabozantinib and regorafenib as category 1 options, with additional consideration for reusing other TKIs or ICIs based on prior exposure and patient characteristics. Ramucirumab is recommended in patients with AFP ≥400 ng/mL, and other targeted therapies (e.g., dostarlimab, selpercatinib, larotrectinib) may be used in biomarker-selected populations (MSI-H/dMMR, RET, NTRK fusions).
LEVIATHAN Study Design
The LEVIATHAN study was a multinational, observational analysis conducted at 26 tertiary centres across Europe, the United States, and Asia using data from the AB-real registry. Out of 1210 patients who received first-line atezolizumab plus bevacizumab (A+B) between May 2018 and October 2024. 230 were eligible for inclusion after applying key criteria: progression on or discontinuation of A+B, Child-Pugh A liver function, and ECOG performance status ≤2. Patients who received locoregional therapies during or between treatment lines, prior CTLA-4 inhibitors, or who died while on A+B were excluded.
Second-line treatment consisted of:
- Lenvatinib (n=125), dosed per REFLECT trial guidelines
- Sorafenib (n=105), dosed per SHARP trial guidelines
Treatment continued until radiologic progression or unacceptable toxicity. Tumor response was assessed every 8–12 weeks per RECIST v1.1, and survival was calculated using Kaplan–Meier methodology.

Read more about Sorafenib (Nexavar): Uses in Cancer, Side Effects, Dosage, Expectations on OncoDaily.
Endpoints
The primary endpoint of the study was overall survival measured from the start of second-line therapy.
Secondary endpoints included:
- Progression-free survival (PFS) from second-line start
- OS from the start of A+B (sequencing benefit)
- Disease control rate (CR + PR + SD)
- Exploratory subgroup analyses by primary vs secondary resistance to A+B
To reduce bias and balance prognostic factors, a propensity score–matched analysis was performed, incorporating ECOG status, AFP level, neutrophil-to-lymphocyte ratio, portal vein thrombosis, and type of resistance to first-line A+B.
LEVIATHAN trial Results
Among the 1210 patients treated with A+B in the first-line setting, 230 were eligible and included in the final analysis. Of these, 125 received lenvatinib and 105 received sorafenib as second-line therapy. Patients in the lenvatinib group generally had more favorable baseline features, including better ECOG performance status and ALBI grade.
After a median follow-up of 29.5 months, treatment with lenvatinib was associated with superior survival outcomes compared with sorafenib.
- Median progression-free survival (PFS): 5.5 months with lenvatinib vs 2.6 months with sorafenib (HR 0.41, p < 0.001)
- Median overall survival (OS) from second-line start: 11.9 vs 7.4 months (HR 0.67, p = 0.018)
- OS from start of A+B (sequencing analysis): 22.4 vs 14.3 months (HR 0.54, p < 0.001)
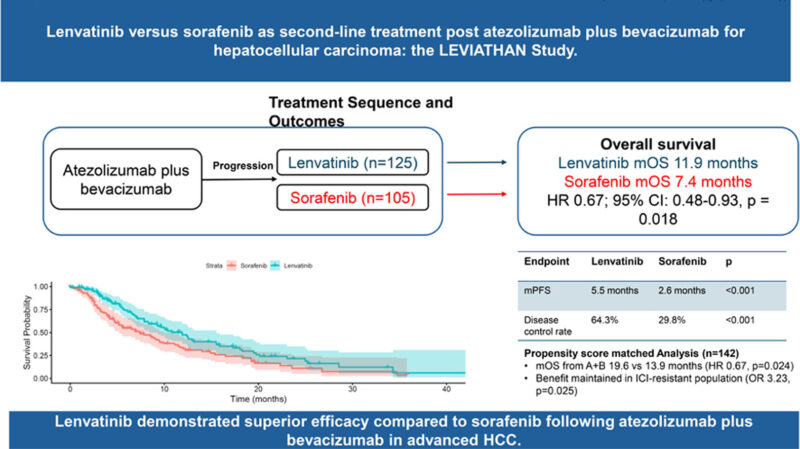
In the propensity score–matched cohort, lenvatinib maintained a consistent benefit:
- Median OS from A+B start: 19.6 vs 13.9 months (HR 0.67, p = 0.024)
- Median PFS: 5.5 vs 2.1 months (HR 0.41, p < 0.001)
- Disease control rate (DCR): 68.9% with lenvatinib vs 32.8% with sorafenib (p < 0.001)
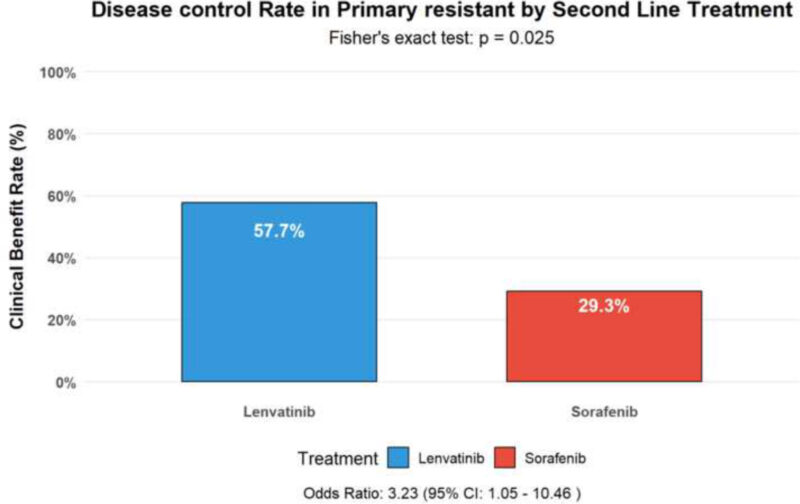
Notably, lenvatinib also showed activity in the subgroup of patients with primary resistance to A+B, achieving disease control in 57.7% compared with 29.3% for sorafenib (OR 3.23, p = 0.025). Multivariate analysis confirmed second-line lenvatinib, AFP ≤ 400 ng/mL, NLR < 3, and absence of portal vein thrombosis as independent predictors of improved survival.
The findings of the LEVIATHAN study were published in JHEP Reports (Elsevier) on September 23, 2025.
Takeaway
The LEVIATHAN study provides the strongest real-world evidence to date that lenvatinib is superior to sorafenib as second-line therapy after A+B, significantly prolonging PFS and OS and improving disease control – even in patients with primary immunotherapy resistance.