Cabozantinib is an oral tyrosine kinase inhibitor used to treat various cancers. It targets multiple pathways that promote tumor growth and angiogenesis.
In March 2025, the FDA expanded its approval to include the treatment of unresectable, locally advanced, or metastatic pancreatic and extrapancreatic neuroendocrine tumors (NETs) in patients aged 12 years and older who have previously received treatment.
This article will explore how cabozantinib works, who it’s for, its side effects, dosing, and what patients can expect during treatment.
Which company produced Cabozantinib?
Cabozantinib (brand name Cabometyx (tablets), Cometriq (capsules)) was developed by Exelixis, Inc., a U.S.-based biotechnology company dedicated to advancing cancer treatment. The company began working on cabozantinib as part of its efforts to create therapies that target the molecular drivers of cancer.
Founded in 1994 and headquartered in Alameda, California, Exelixis focuses on discovering and developing targeted therapies for cancer. Its mission is to improve patient outcomes by delivering innovative treatments, and Cabozantinib remains a key part of its growing oncology portfolio.
While Exelixis handles U.S. marketing, it partners with Ipsen for distribution outside the U.S. (excluding Japan) and with Takeda in Japan. These global collaborations have helped expand access to cabozantinib and support further research into its cancer-fighting potential.
How does Cabozantinib work?
Cabozantinib is a tyrosine kinase inhibitor (TKI). To understand how cabozantinib works, it helps to know a little about how cancer grows. Inside your body, cells use tiny switches called tyrosine kinases to send signals—like instructions—to grow, divide, or form new blood vessels. Normally, these signals are carefully controlled. But in many cancers, these switches get stuck in the “on” position. The result? Tumors grow out of control and start building their own blood supply to fuel their growth.
This is where Cabozantinib comes in. It’s a targeted cancer drug that blocks several of these overactive switches, including ones called VEGFR, MET, and AXL. By doing this, cabozantinib helps:
- Slow down cancer cell growth
- Cut off the blood supply to the tumor
- Stop the cancer from spreading
Think of it like flipping off the circuit breakers that cancer cells depend on. Without those constant “grow” and “spread” signals, the tumor loses its power source.
Photo from researchgate.net
What Cancers Is Cabozantinib Approved to Treat?
Cabozantinib is a targeted therapy approved by the FDA for several types of cancer, helping patients with advanced or difficult-to-treat conditions. Here’s a breakdown of the cancers for which cabozantinib is FDA-approved:
Advanced Renal Cell Carcinoma (RCC): Approved in April 2016 for patients with advanced renal cell carcinoma (RCC) who have previously been treated with other anti-angiogenic therapies. Cabozantinib has shown significant efficacy in improving progression-free survival (PFS) in these patients.
Hepatocellular Carcinoma (HCC): In January 2019, the FDA approved cabozantinib for patients with hepatocellular carcinoma (HCC) who have previously been treated with sorafenib. This approval was based on positive clinical trial results showing improved overall survival (OS) and PFS in patients with advanced HCC.
Differentiated Thyroid Cancer (DTC): Cabozantinib received FDA approval in September 2021 for differentiated thyroid cancer (DTC) patients aged 12 and older who have locally advanced or metastatic disease and have progressed after radioactive iodine treatment. This approval was granted based on clinical evidence of significant tumor shrinkage.
Neuroendocrine Tumors (NETs): In March 2025, the FDA approved cabozantinib for unresectable, locally advanced, or metastatic neuroendocrine tumors (NETs), including both pancreatic neuroendocrine tumors (pNET) and extra-pancreatic neuroendocrine tumors (epNET), in patients aged 12 and older. This approval was based on data showing improved PFS and response rates in these challenging cancers.
Cabozantinib’s multiple FDA approvals reflect its broad potential in treating various cancer types, offering hope for patients with advanced disease and those who have not responded to other treatments. With its ability to target key pathways involved in cancer growth, cabozantinib is a critical option for managing these hard-to-treat cancers.
What research is behind the approval?
Cabometyx is approved by the FDA to treat several types of cancer, including advanced renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), and differentiated thyroid cancer (DTC). These approvals are based on strong clinical evidence from large phase 3 trials that demonstrated improved survival outcomes and disease control.
Below, we’ll explore each approval in detail, highlighting the key clinical trials and research that led to cabozantinib’s use in these cancers.
Cabozantinib for Renal Cell Carcinoma
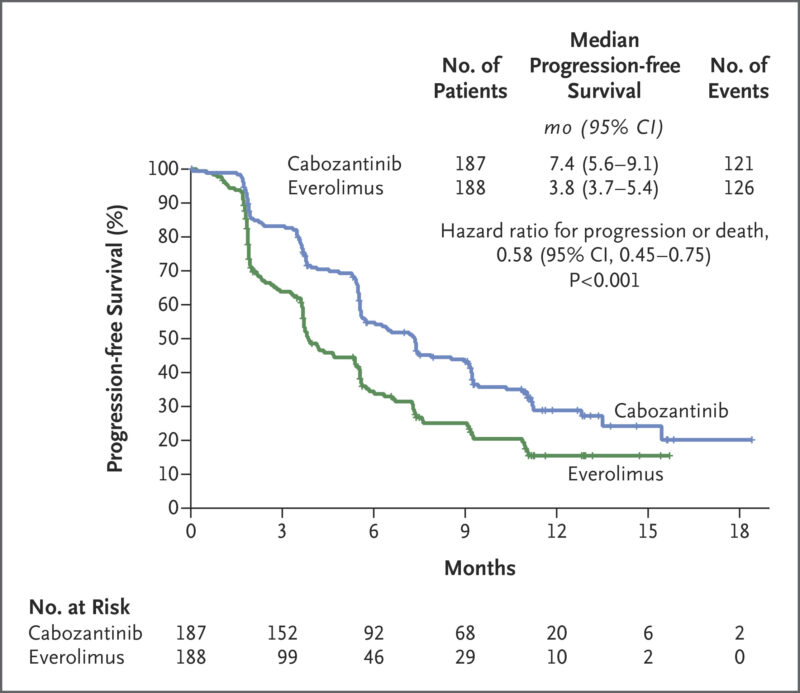
In the METEOR trial, published in NEJM in 2015, cabozantinib demonstrated significant improvements over everolimus in treating renal cell carcinoma (RCC) that had progressed after VEGFR-targeted therapy. A total of 658 patients participated in the study, receiving either cabozantinib (60 mg daily) or everolimus (10 mg daily).
The results showed
- Progression-Free Survival (PFS): Cabozantinib increased PFS to 7.4 months, compared to 3.8 months with everolimus.
- Objective Response Rate: 21% of cabozantinib patients responded, compared to just 5% with everolimus.
- Overall Survival: Cabozantinib extended overall survival, with a hazard ratio for death of 0.67.
- Safety: While side effects were common, 60% of cabozantinib patients had dose reductions, and 9% discontinued treatment due to side effects.
These results confirm cabozantinib’s superior efficacy in advanced RCC, offering longer survival and a higher response rate compared to everolimus.
On January 22, 2021, the FDA approved the combination of Cabozantinib and nivolumab (Opdivo) as a first-line treatment for advanced renal cell carcinoma (RCC).
This approval was based on results from the CHECKMATE-9ER trial, which showed the combo significantly improved progression-free survival, overall survival, and response rates compared to sunitinib. Median PFS was 16.6 months vs. 8.3 months, with a response rate of 55.7% for the combination.
Common side effects included diarrhea, fatigue, liver toxicity, skin reactions, and hypertension. The recommended doses are nivolumab 240 mg IV every 2 weeks or 480 mg every 4 weeks, with Cabozantinib 40 mg orally daily.
Cabozantinib for Hepatocellular Carcinoma
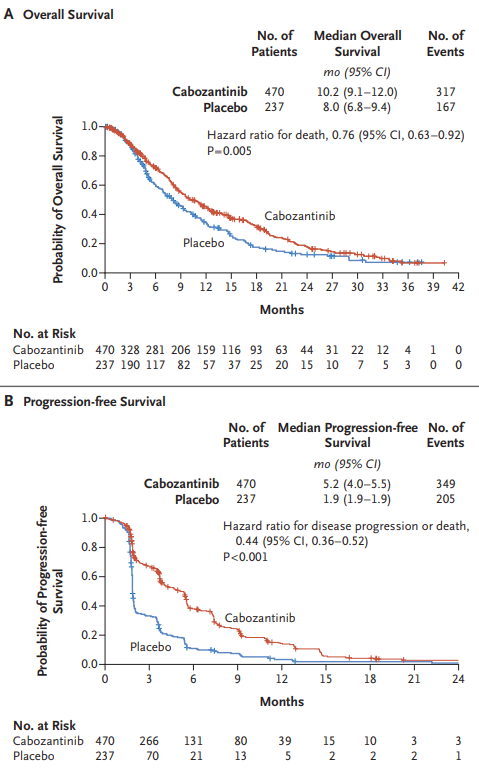
The CELESTIAL trial, published in NEJM in 2018, evaluated cabozantinib (60 mg daily) versus placebo in patients with advanced hepatocellular carcinoma (HCC) who had previously been treated with sorafenib. The study involved 707 patients and aimed to assess overall survival (OS), progression-free survival (PFS), and objective response rate (ORR).
The results showed:
- Overall Survival (OS): Cabozantinib significantly extended OS, with a median of 10.2 months, compared to 8.0 months for placebo (hazard ratio 0.76, P=0.005).
- Progression-Free Survival (PFS): Cabozantinib improved PFS to 5.2 months, versus 1.9 months with placebo (hazard ratio 0.44, P<0.001).
- Objective Response Rate: The ORR was 4% for cabozantinib, compared to less than 1% for placebo (P=0.009).
- Safety: High-grade adverse events were more common with cabozantinib, affecting 68% of patients, compared to 36% in the placebo group. The most frequent were palmar-plantar erythrodysesthesia, hypertension, and fatigue.
These results highlight cabozantinib’s potential to improve survival and disease control in advanced HCC, offering a promising option for patients who have failed previous treatments.
Cabozantinib for Thyroid Cancer
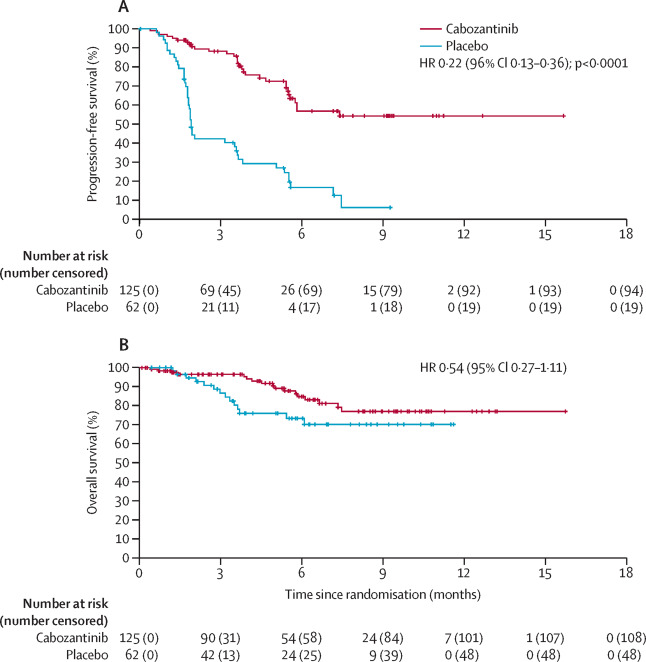
In 2021, results published in The Lancet Oncology showed that cabozantinib significantly improved outcomes in patients with radioiodine-refractory differentiated thyroid cancer (DTC) who had previously received VEGFR-targeted therapies like lenvatinib or sorafenib. These patients typically have aggressive disease and limited treatment options.
The phase 3 COSMIC-311 trial enrolled 187 patients and randomized them 2:1 to cabozantinib (60 mg daily) or placebo. The study met its primary endpoint: median progression-free survival (PFS) was not reached in the cabozantinib group versus 1.9 months with placebo (HR 0.22; p<0.0001). Although the confirmed objective response rate was 15% with cabozantinib and 0% with placebo, it did not meet the predefined significance level.
Grade 3 or 4 adverse events occurred in 57% of patients receiving cabozantinib, with the most common being hand-foot syndrome, hypertension, and fatigue. Despite the toxicity, the data support cabozantinib as an effective treatment option for patients with advanced, treatment-resistant DTC.
Cabozantinib for differentiated pancreatic neuroendocrine tumors
The CABINET trial was a Phase 3 study evaluating Cabozantinib in patients with advanced pancreatic (pNET) and extra-pancreatic (epNET) neuroendocrine tumors (NETs). The trial enrolled 298 patients, randomly assigned to receive Cabometyx or placebo. The primary endpoint was progression-free survival (PFS), with secondary endpoints of overall response rate (ORR) and overall survival (OS).
Cabozantinib significantly improved PFS in both pNET and epNET cohorts. In pNET, median PFS was 13.8 months with cabozantinib versus 3.3 months with placebo (HR: 0.22, p<0.0001), and ORR was 18% vs. 0%. In epNET, median PFS was 8.5 months vs. 4.2 months (HR: 0.40, p<0.0001), with ORR of 5% vs. 0%.
Although OS data were immature due to crossover from placebo, the study confirmed cabozantinib’s potential for treating progressive NETs. The FDA approved cabozantinib for treating unresectable, advanced pNET and epNET on March 26, 2025.
Read more about Cabozantinib approval for unresectable, locally advanced or metastatic well-differentiated pNET and well-differentiated epNET on OncoDaily.
Learn more about Pancreatic Neuroendocrine Tumors Overview: Comprehensive 2025 Insights on OncoDaily.
Cabozantinib side effects and its management
Cabozantinib is a tyrosine kinase inhibitor used to treat various cancers, including thyroid, kidney, and liver cancers. While effective, it can cause a range of side effects, from mild to severe. Understanding these side effects and their management is crucial for patients undergoing treatment.
Common side effects
The most frequently reported side effects of cabozantinib include fatigue, diarrhea, nausea, hypertension, stomatitis (mouth sores), and elevated liver enzymes (AST/ALT). Fatigue affected up to 79% of patients in clinical trials, while diarrhea occurred in more than 70%. Hand-foot syndrome (PPE), characterized by redness and pain in the palms and soles, is another common and potentially dose-limiting side effect. These symptoms are usually manageable with supportive care and dose modifications.
Patients may also experience decreased appetite, weight loss, skin rash, hair color changes, and changes in taste (dysgeusia). Metabolic abnormalities such as hypocalcemia, hypophosphatemia, and hypoalbuminemia are frequent and often monitored with routine lab tests.
Less Common and Serious Side Effects
Less commonly, cabozantinib may cause thromboembolic events, gastrointestinal perforation, fistula formation, and bleeding complications. It can also affect thyroid and glucose metabolism, leading to hypothyroidism or hyperglycemia. Some patients develop blood count abnormalities, including neutropenia, thrombocytopenia, and anemia, which may increase the risk of infection or bleeding.
High-grade (Grade 3/4) toxicities include severe diarrhea, hypertension, liver enzyme elevations, fatigue, and PPE. In trials, up to 28% experienced grade 3 hypertension, while grade 3 PPE occurred in up to 17% of patients.

Managing Cabozantinib Side Effects
Effective side effect management involves regular monitoring, early intervention, and patient education. Blood pressure should be checked frequently, and antihypertensives started as needed. GI issues can be managed with anti-diarrheal and anti-nausea medications. Mouth rinses, oral hygiene, and topical treatments help with stomatitis. For hand-foot syndrome, moisturizers, avoiding friction, and dose interruptions are recommended.
Lab abnormalities and blood count changes require routine monitoring and, in some cases, supplementation or medical management. Dose reductions (typically in 20 mg steps) are advised for persistent moderate or severe side effects. Treatment may be paused or discontinued if side effects become unmanageable.
What is the Recommended Dosage of Cabozantinib?
Cabozantinib is an oral tyrosine kinase inhibitor used to treat several advanced cancers, including thyroid cancer, renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), and neuroendocrine tumors (NETs). It is marketed in two forms: Cometriq capsules (20 mg, 80 mg) and Cabometyx tablets (20 mg, 40 mg, 60 mg). The choice between them depends on the cancer type.
For medullary thyroid cancer, Cometriq is given at 140 mg daily. For other cancers like differentiated thyroid cancer, advanced RCC, HCC, and neuroendocrine tumors, Cabometyx is typically prescribed at 60 mg daily, taken until disease progression or unacceptable side effects. In advanced RCC, cabozantinib may also be combined with nivolumab (40 mg cabozantinib daily plus nivolumab infusions every 2 or 4 weeks).
If side effects occur, such as diarrhea, high blood pressure, liver enzyme elevations, or hand-foot syndrome, the dosage can be reduced stepwise (from 60 mg to 40 mg, then 20 mg). The drug should be temporarily stopped for severe reactions and restarted at a lower dose once symptoms improve.
Learn more about Kidney Cancer: Symptoms ,Causes, Stages, Diagnosis and Treatment on OncoDaily.
How is Cabozantinib administered?
Cabozantinib is available in two forms: Cabometyx tablets and Cometriq capsules, each approved for different uses. It’s important not to substitute one for the other. When taking Cabometyx tablets, they should be taken on an empty stomach, at least 1 hour before or 2 hours after eating. For Cometriq capsules, take them without food, ensuring there’s a 2-hour gap before and 1 hour after eating. Both tablets and capsules should be swallowed whole; do not crush, chew, or split them.
While undergoing treatment, avoid grapefruit and St. John’s wort, as these can affect the medication’s effectiveness. If you miss a dose, do not take it within 12 hours of the next dose. Store the medication at room temperature (20–25°C or 68–77°F), allowing for some fluctuation between 15–30°C (59–86°F).
What to Avoid During Cabozantinib Treatment?
Cabozantinib is metabolized by CYP3A4, so it may interact with CYP3A4 inhibitors and inducers. Strong CYP3A4 inhibitors should be avoided, as they can increase cabozantinib levels, raising the risk of adverse effects. If coadministration is unavoidable, the cabozantinib dose should be reduced according to dosing guidelines. Similarly, moderate or strong CYP3A4 inducers should be avoided, as they can reduce cabozantinib’s effectiveness. If coadministration is necessary, the dosage may need to be increased.
Pregnancy and Lactation
Cabozantinib may cause fetal harm, so it should be avoided during pregnancy. Pregnancy status must be confirmed before treatment begins, and women of reproductive potential are advised to use effective contraception during treatment and for 4 months after the final dose. The drug may also impair fertility in both men and women.
The effects of cabozantinib on lactation are not fully known. However, due to potential risks to a breastfed infant, women are advised not to breastfeed during treatment and for 4 months after the final dose.
Read more about Neuroendocrine Tumors: The Silent Cancer on the Rise – Breakthroughs in Diagnosis, Treatment, Survival and Latest 2025 Reaserches on OncoDaily.
Cabozantinib effectiveness over time
Cabozantinib has demonstrated sustained effectiveness over time across various cancers, with both clinical trials and real-world studies supporting its long-term benefits.
In 2024, updated results from the CheckMate 9ER trial were published on PubMed, confirming long-term benefits of cabozantinib plus nivolumab as a first-line treatment for advanced renal cell carcinoma (aRCC).
After 44 months of follow-up, the combination continued to outperform sunitinib, with:
- Median progression-free survival (PFS) of 16.6 vs. 8.4 months
- Median overall survival (OS) of 49.5 vs. 35.5 months
- Objective response rate (ORR) of 56% vs. 28%, with 13% achieving a complete response
Benefits were consistent across all IMDC risk groups. Side effects remained manageable and similar to earlier reports.
These results further support cabozantinib plus nivolumab as a durable and effective first-line option for aRCC.
Ongoing trials with Cabozantinib
Cabozantinib is currently being evaluated in multiple clinical trials across different cancer types. These trials aim to assess its effectiveness, safety, and potential for improving patient outcomes when used alone or in combination with other therapies.
Phase II Trial (NCT06900595) – Cabozantinib + Cemiplimab for Adrenocortical Cancer: This trial is evaluating the combination of cabozantinib and cemiplimab in patients with locally advanced, unresectable, recurrent, or metastatic adrenocortical cancer. The primary goal is to determine if the combination improves progression-free survival (PFS) compared to cabozantinib alone, using RECIST v1.1 criteria.
Phase I/II Trial (NCT06811116) – Sapanisertib + Cabozantinib for Metastatic Liver Cancer: This trial investigates the combination of sapanisertib and cabozantinib in patients with metastatic liver cancer that has a β-catenin mutation. The study aims to determine the optimal dose of sapanisertib and evaluate its effect on progression-free survival (PFS) compared to cabozantinib alone. Secondary objectives include assessing response rates, overall survival, safety, and pharmacokinetics.
Phase II, Single-Arm Study (NCT06535737) – Cabozantinib for Advanced Hepatocellular Carcinoma (HCC): This phase II, single-arm study examines cabozantinib in patients with advanced hepatocellular carcinoma (HCC) who have previously received atezolizumab and bevacizumab. The goal is to assess the efficacy and safety of cabozantinib in this patient population, where there are currently no proven therapies after treatment with atezolizumab and bevacizumab.
You can read about Neuroendocrine Lung Tumors: Types, Symptoms, Diagnosis, Treatment and 2025 updates on OncoDaily.
FAQ
What cancers are treated with Cabozantinib?
Cabozantinib is approved for the treatment of advanced renal cell carcinoma (kidney cancer), hepatocellular carcinoma (liver cancer), and medullary thyroid cancer.
Is Cabozantinib a type of chemotherapy?
No, it is not a traditional chemotherapy. It is a targeted therapy known as a tyrosine kinase inhibitor (TKI), which blocks signals that tumors use to grow and form new blood vessels.
What are the most common side effects of Cabozantinib?
The most common side effects include diarrhea, fatigue, decreased appetite, weight loss, nausea, hand-foot syndrome (palmar-plantar erythrodysesthesia), and high blood pressure.
Can Cabozantinib be taken with food?
Cabometyx should be taken on an empty stomach—at least 1 hour before or 2 hours after eating—to ensure proper absorption and effectiveness.
What should you do if you miss a dose of Cabometyx?
If you miss a dose and it's less than 12 hours until your next one, skip the missed dose and take the next one at the regular time. Do not take two doses at once.
Can Cabozantinib be used in combination with immunotherapy?
Yes, Cabozantinib is sometimes used in combination with immunotherapy agents, such as nivolumab, particularly in the treatment of advanced kidney cancer.


