Pancreatic neuroendocrine tumors (PNETs), also known as islet cell tumors, are a rare form of cancer originating from the hormone-producing cells of the pancreas. Unlike the more common pancreatic adenocarcinomas that arise from exocrine cells, PNETs develop in the endocrine cells responsible for regulating vital bodily functions through hormone secretion.
You Can Read More About Neuroendocrine Tumors on Oncodaily
What Are PNETs?
The pancreas serves dual roles: its exocrine component produces digestive enzymes, while the endocrine component consists of clusters of cells called islets of Langerhans. These islets produce hormones such as insulin and glucagon, which are crucial for maintaining blood sugar levels. PNETs emerge from these hormone-producing cells and can be classified into two main categories:
- Functioning PNETs: These tumors actively secrete hormones, leading to specific clinical syndromes. For instance, insulinomas produce excessive insulin, causing low blood sugar levels, while gastrinomas secrete gastrin, leading to Zollinger-Ellison syndrome, characterized by severe stomach ulcers and diarrhea.
- Nonfunctioning PNETs: These tumors do not produce excess hormones and may not present noticeable symptoms until they grow large or metastasize.
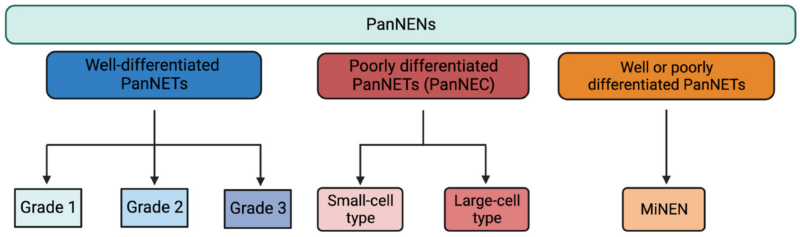
The World Health Organization (WHO) further classifies PNENs based on histological differentiation and proliferative rate into well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs).
Causes and Risk Factors of Pancreatic Neuroendocrine Tumors
Pancreatic neuroendocrine tumors (PNETs) are rare and complex cancers that arise from the hormone-producing cells of the pancreas. While the exact cause of PNETs is still not fully understood, research has identified several potential risk factors and underlying mechanisms that may contribute to their development.
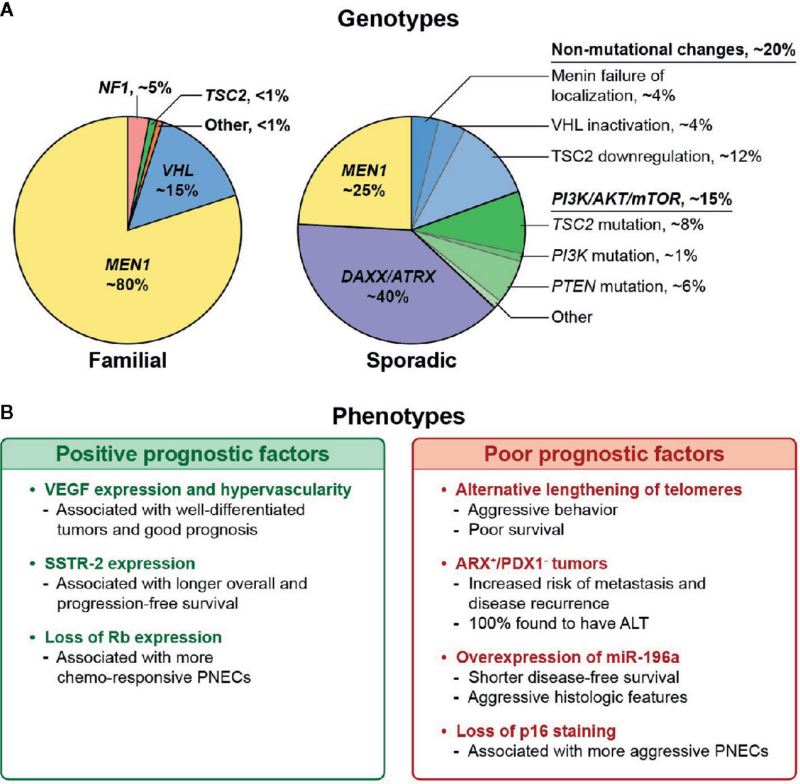
Genetic Syndromes
A significant number of PNET cases—especially in younger individuals or those with multiple tumors—are linked to inherited genetic conditions. These syndromes increase the risk of developing tumors in the pancreas and other organs.
- Multiple Endocrine Neoplasia type 1 (MEN1): This is the most common inherited condition associated with PNETs. It is caused by mutations in the MEN1 gene and increases the risk of tumors in the pancreas, parathyroid, and pituitary glands.
- Von Hippel-Lindau (VHL) Syndrome: Caused by mutations in the VHL gene, this condition raises the risk of PNETs and other tumors in the kidneys, adrenal glands, and central nervous system.
- Neurofibromatosis Type 1 (NF1): Though less common, NF1 is associated with the development of NETs in the gastrointestinal tract.
- Tuberous Sclerosis Complex (TSC): This rare disorder, caused by mutations in the TSC1 or TSC2 genes, has also been linked to pancreatic NETs in some cases.
Family History
Even in the absence of a known genetic syndrome, having a family history of PNETs or other endocrine tumors may increase your risk. Family history may reflect inherited predispositions that are not yet fully identified.
Age and Gender
PNETs can occur at any age but are most commonly diagnosed between the ages of 40 and 60. Some studies suggest a slightly higher incidence in males, although the difference is modest.(World Health Organisation)
Environmental and Lifestyle Factors
Unlike other forms of pancreatic cancer, there is limited evidence linking smoking, alcohol, or dietary factors directly to PNETs. However, chronic inflammation of the pancreas (pancreatitis) has been loosely associated with a higher risk, although this is more established in exocrine pancreatic cancers.
Hormonal Influences
Some researchers believe that excessive or chronic stimulation of hormone-producing cells may contribute to the development of functional PNETs. However, this theory is still under investigation, and no definitive causal link has been established.
Symptoms of Pancreatic Neuroendocrine Tumors
Pancreatic neuroendocrine tumors (PNETs) can present with a wide range of symptoms—or sometimes none at all. The signs and symptoms largely depend on whether the tumor is functioning (producing excess hormones) or non-functioning (not producing hormones). Here’s an overview of what patients might experience:
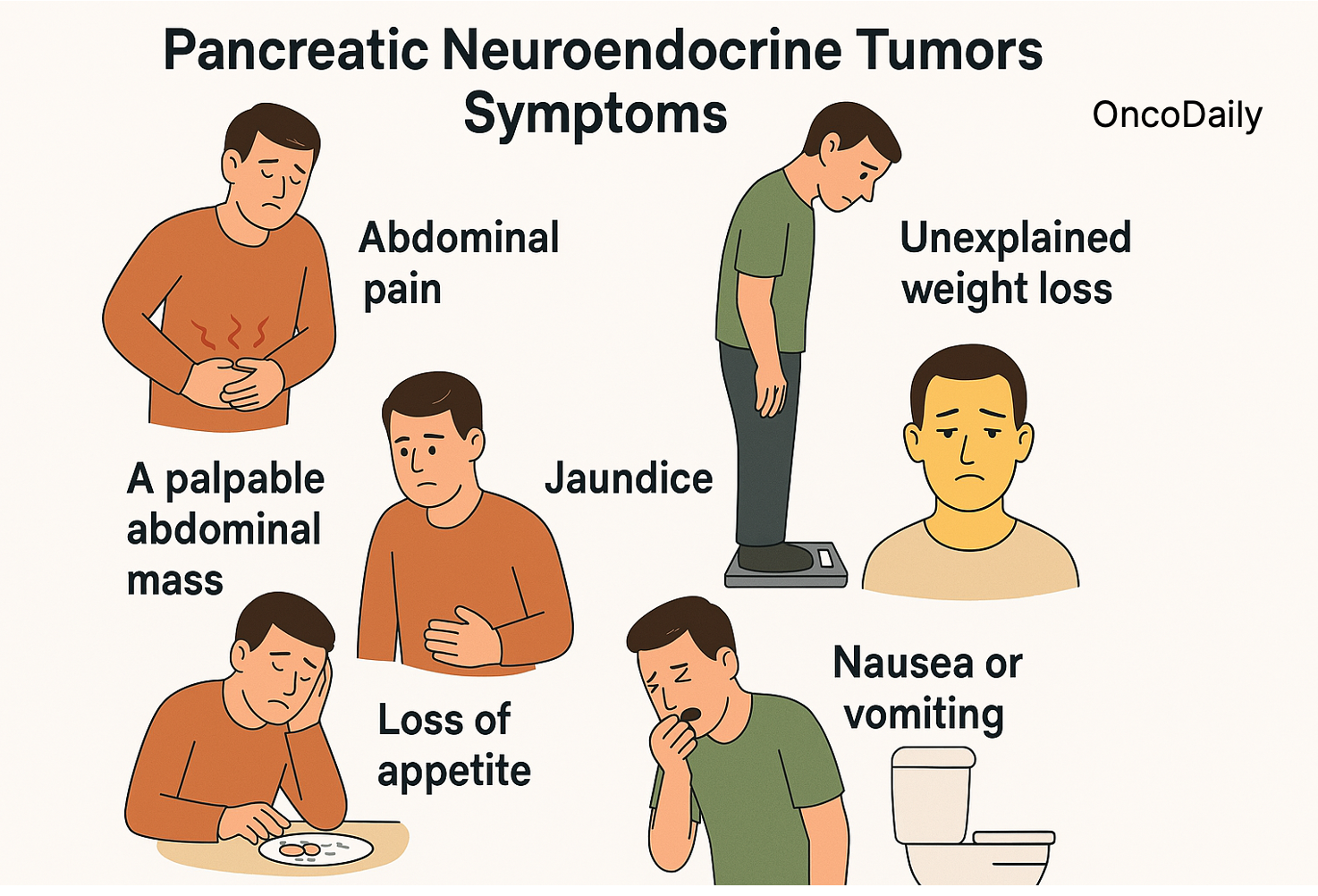
Non-Functioning PNETs
These tumors do not secrete hormones in excess, so symptoms are typically related to the tumor’s size, growth, or spread to nearby structures. Non-functioning tumors are often diagnosed later because symptoms can be vague or absent.
Common symptoms include:
- Abdominal pain or discomfort (due to tumor growth or pressure on nearby organs)
- Unexplained weight loss
- A palpable abdominal mass
- Jaundice (yellowing of the skin and eyes, if the tumor blocks bile ducts)
- Loss of appetite
- Nausea or vomiting
Functioning PNETs
These tumors produce excess hormones, leading to distinct clinical syndromes. The symptoms depend on the type of hormone being overproduced:
- Insulinoma (produces insulin): Causes episodes of low blood sugar, which may result in confusion, weakness, fainting, dizziness, or even seizures.
- Gastrinoma (produces gastrin): Leads to Zollinger-Ellison syndrome, characterized by recurrent peptic ulcers, acid reflux, abdominal pain, and diarrhea.
- Glucagonoma (produces glucagon): Associated with high blood sugar, weight loss, a distinctive rash (necrolytic migratory erythema), and anemia.
- VIPoma (produces vasoactive intestinal peptide): Causes profuse watery diarrhea, dehydration, flushing, and low levels of potassium.
- Somatostatinoma (produces somatostatin): Leads to diabetes, gallstones, weight loss, and diarrhea.
Diagnosis of Pancreatic Neuroendocrine Neoplasms
Diagnosing pancreatic neuroendocrine neoplasms (PNENs) involves a combination of clinical evaluation, biochemical tests, imaging, and histopathological confirmation. Because these tumors are often indolent and can be asymptomatic, they are sometimes discovered incidentally during imaging for other conditions.
Clinical Evaluation
Functioning PNENs may present with symptoms related to hormone overproduction. For example, insulinomas may cause hypoglycemia, while gastrinomas can lead to peptic ulcers. Non-functioning PNENs, which are more common, typically present with nonspecific symptoms like abdominal pain, weight loss, or jaundice—or may be found incidentally.
Biochemical Testing
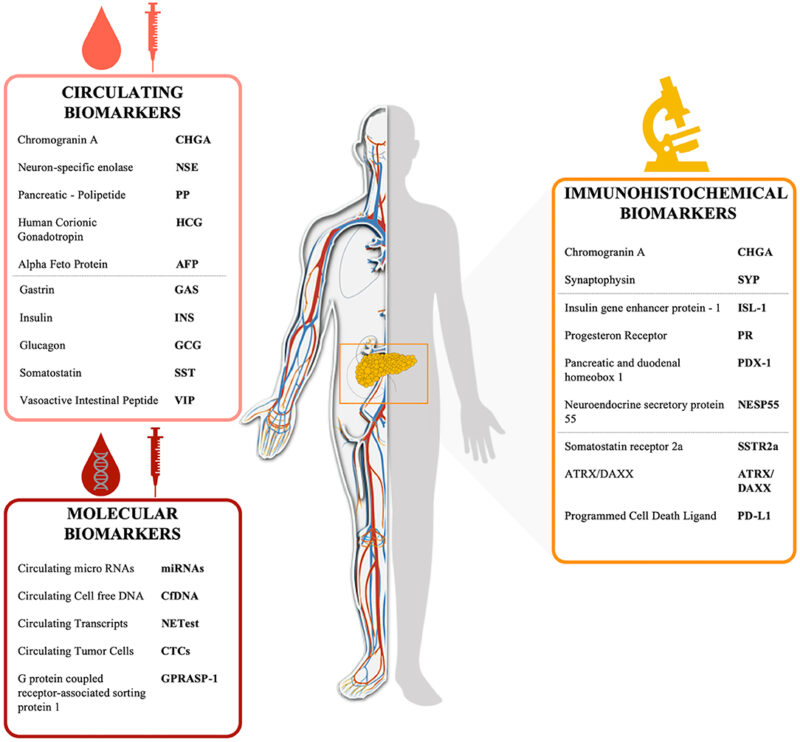
Blood and urine tests are essential for identifying hormone-producing tumors:
- Insulin, C-peptide, and glucose levels for insulinomas
- Gastrin levels for gastrinomas
- Chromogranin A (CgA) as a general neuroendocrine tumor marker
- Pancreatic polypeptide, glucagon, and VIP (vasoactive intestinal peptide) for other rare functioning tumors
Imaging Techniques
High-quality imaging is crucial to determine the location, size, and spread of PNENs:
- Multiphasic contrast-enhanced CT: First-line imaging for pancreatic lesions
- MRI with gadolinium: Especially effective in detecting liver metastases
- Endoscopic ultrasound (EUS): Highly sensitive for small pancreatic tumors and allows for fine needle aspiration (FNA) biopsy
- Somatostatin receptor imaging: Such as Ga-68 DOTATATE PET/CT, which is highly sensitive for well-differentiated NETs
Histological Confirmation
Biopsy is needed for definitive diagnosis and grading. EUS-guided FNA or core biopsy is commonly used. The tumor’s grade is determined by mitotic count and the Ki-67 index:
- G1: Ki-67 index <3%
- G2: 3–20%
- G3: >20% (includes poorly differentiated carcinomas)
Molecular and Genetic Testing
In select cases, especially if hereditary syndromes like MEN1 are suspected, genetic testing may be warranted. Immunohistochemistry and next-generation sequencing can help identify prognostic and therapeutic biomarkers.
How Pancreatic Neuroendocrine Tumors Are Treated?
Pancreatic neuroendocrine tumors, or PNETs, are rare tumors that require specialized care. Depending on how aggressive the tumor is, whether it makes hormones, and if it has spread to other organs, doctors will tailor the treatment plan for each person. Here’s an easy-to-understand overview of the available treatment options:
Surgery
Surgery is often the best option if the tumor is caught early and hasn’t spread. In some cases, only the tumor is removed, while in others, part of the pancreas may need to be taken out. For larger or more complex tumors, a Whipple surgery may be required. Even when the tumor has spread, surgeons might remove part of it to help relieve symptoms and improve quality of life.
Somatostatin Analogs (SSAs)
Some PNETs produce hormones that cause uncomfortable symptoms like low blood sugar, ulcers, or diarrhea. Drugs called somatostatin analogs—such as octreotide and lanreotide—can help control these symptoms. These medications also help slow tumor growth.
Targeted Therapy
Targeted therapy is a major advancement in the treatment of pancreatic neuroendocrine tumors (PNETs), offering a more focused approach than traditional chemotherapy. While chemotherapy affects both healthy and cancerous cells, targeted therapies are designed to interfere with specific molecules or pathways that cancer cells use to grow, divide, and spread. This more refined strategy helps slow tumor progression while generally causing fewer side effects.
- Everolimus (Afinitor): The efficacy of everolimus in treating advanced pancreatic neuroendocrine tumors was established in the RADIANT-3 trial. This Phase III study demonstrated that everolimus significantly improved progression-free survival (PFS) compared to placebo (Yao JC, et al. Everolimus for advanced pancreatic neuroendocrine tumors.New England Journal of Medicine. 2011).
- Sunitinib (Sutent): A pivotal Phase III trial confirmed that sunitinib also significantly extended PFS in patients with advanced PNETs. The study reported a median PFS of 11.4 months for sunitinib versus 5.5 months for placebo (Raymond E, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors.New England Journal of Medicine. 2011).
Peptide Receptor Radionuclide Therapy
Peptide Receptor Radionuclide Therapy (PRRT) has become a vital treatment for patients with advanced pancreatic neuroendocrine tumors (PNETs) that express somatostatin receptors. PRRT works by using radiolabeled peptides—most notably Lutetium-177 dotatate (Lu-177)—that bind to somatostatin receptors on tumor cells and deliver targeted radiation to kill cancer cells, minimizing damage to surrounding healthy tissues.
This approach is often described as a “heat-seeking missile” because the radiolabeled drug specifically targets tumor cells, sparing most normal tissues. It has been particularly useful for patients with well-differentiated, unresectable, or metastatic NETs that are no longer responding to standard therapies.
Clinical evidence supporting PRRT’s effectiveness was established in the landmark NETTER-1 trial, a multicenter, randomized phase III study. The trial demonstrated that patients with midgut NETs treated with Lu-177 dotatate plus octreotide had a progression-free survival (PFS) of 28.4 months, compared to 8.5 months in the control group receiving high-dose octreotide alone (Strosberg J, et al. N Engl J Med. 2017). Although the trial primarily focused on midgut NETs, its results have guided clinical use in other somatostatin receptor-positive tumors, including pancreatic NETs.
In clinical practice, PRRT is most suitable for patients whose tumors demonstrate strong uptake on Ga-68 DOTATATE PET/CT scans. This ensures that the therapy will specifically target cancer cells expressing somatostatin receptors. PRRT has been shown to not only prolong disease control but also improve quality of life in many cases (National Cancer Institute, 2024).
Chemotherapy for More Aggressive Cases
A randomized Phase II trial demonstrated that the combination of capecitabine and temozolomide (CAPTEM) was significantly more effective than temozolomide alone in treating advanced pancreatic neuroendocrine tumors. The study found a median progression-free survival (PFS) of 22.7 months for CAPTEM compared to 14.4 months for temozolomide monotherapy.Kunz PL, Graham NT, Catalano PJ, et al. Randomized study of temozolomide or temozolomide plus capecitabine in patients with advanced pancreatic neuroendocrine tumors: A National Cancer Institute Alliance Trial (A021202). J Clin Oncol. 2022.
Treatments for Tumors That Spread to the Liver
Liver metastases are common in PNETs and are often managed with interventional radiologic therapies. Salem et al. (2012) demonstrated that Yttrium-90 (90Y) radioembolization for hepatic metastases of neuroendocrine tumors provided effective tumor control and prolonged survival in many patients (Salem R, et al. Cancer. 2012).
In more advanced or refractory cases, liver transplantation may be considered. A European multicenter study by Mazzaferro et al. (2016) found that while liver transplantation was a viable option, patients with PNETs had lower 5-year survival compared to those with other primary tumor sites (Mazzaferro V, et al. Ann Surg. 2016).
What About Immunotherapy?
Immunotherapy is a groundbreaking type of cancer treatment that uses the body’s own immune system to recognize and destroy cancer cells. It has transformed the treatment landscape for several cancers like melanoma, lung, and kidney cancer. However, in pancreatic neuroendocrine tumors (PNETs), the role of immunotherapy is still evolving and remains under active investigation.
Unlike some aggressive cancers that respond well to immune-based treatments, PNETs are generally slow-growing and don’t often trigger strong immune responses. This makes them less responsive to standard immunotherapies like immune checkpoint inhibitors. These drugs—such as nivolumab (anti-PD-1) and pembrolizumab—work by blocking proteins that cancer cells use to hide from the immune system. In early studies, checkpoint inhibitors have shown limited success in PNETs, particularly in well-differentiated tumors. However, some high-grade or poorly differentiated neuroendocrine carcinomas may benefit more, especially if they express high levels of PD-L1 or have a high tumor mutational burden.
Researchers are now exploring combination strategies to improve outcomes. This includes using immunotherapy alongside other treatments like peptide receptor radionuclide therapy (PRRT) or targeted therapies. These combinations may enhance the immune system’s ability to detect and destroy tumor cells. For example, damage caused by PRRT may expose more tumor antigens, making immunotherapy more effective.
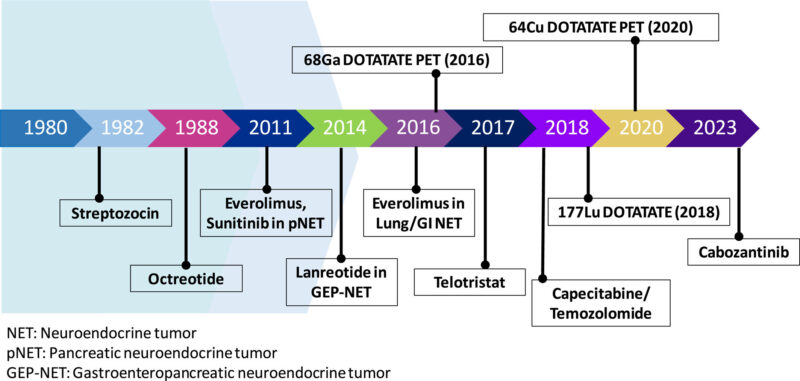
Pancreatic Neuroendocrine Tumors Treatment Advances: Recent Approvals & Clinical Trials
The therapeutic landscape for pancreatic neuroendocrine tumors (PNETs) has recently expanded with new treatment approvals and promising clinical trials, offering renewed hope for patients with this challenging disease.
FDA Approval of Cabozantinib for PNETs
IThe Phase III CABINET trial demonstrated that cabozantinib significantly improves progression-free survival in patients with advanced pancreatic neuroendocrine tumors (pNETs). Patients receiving cabozantinib had a median PFS of 13.8 months vs. 4.4 months with placebo (HR, 0.23; NEJM, 2024). These results led to its FDA approval in March 2025 for previously treated, advanced well-differentiated pNETs.

You Can Read More About Cabozantinib on Oncodaily
Peptide Receptor Radionuclide Therapy
Peptide receptor radionuclide therapy (PRRT) has emerged as a targeted treatment for metastatic, well-differentiated PNETs. Lutetium Lu 177 dotatate (Lutathera) delivers targeted radiation to tumor cells expressing somatostatin receptors. The NETTER-1 trial demonstrated a 54.4% increase in estimated PFS at 20 months for patients treated with Lutathera in combination with somatostatin analogs compared to somatostatin analogs alone (National Cancer Institute).
Research into novel therapies for PNETs is ongoing
Surufatinib Combined with Immunotherapy: A clinical trial is investigating the combination of surufatinib, a targeted therapy, with tislelizumab, an immunotherapy agent, for neuroendocrine tumors in the lungs, pancreas, and gastrointestinal tract. This approach aims to enhance the immune system’s ability to recognize and attack tumor cells.
Personalized Cancer Vaccines: Early-phase trials are exploring personalized mRNA vaccines designed to elicit an immune response against specific tumor antigens in pancreatic cancer patients. Preliminary results have shown that some patients remained cancer-free for extended periods post-treatment.
KRAS Inhibitors: Given that approximately 90% of pancreatic cancers harbor KRAS mutations, targeted therapies like daraxonrasib are under investigation. Early trials have shown that patients with stage-four pancreatic cancer experienced significant responses, with extended survival times (UCLA Health).
Prognosis of Pancreatic Neuroendocrine Tumors
The prognosis for PNETs varies widely depending on tumor grade, differentiation, metastasis status, and response to therapy.
Well-differentiated, low-grade tumors (Grade 1 or Grade 2) are typically slow-growing and associated with favorable outcomes. In cases where tumors are localized and surgically resected, the 5-year overall survival can exceed 80%. This statistic is reported in the National Institutes of Health (NIH) Surveillance, Epidemiology, and End Results (SEER) Program.
High-grade or poorly differentiated neuroendocrine carcinomas (Grade 3) are aggressive, and the prognosis is generally worse. Studies indicate a 5-year survival rate below 30% for patients with metastatic high-grade tumors (Yao et al., Journal of Clinical Oncology, 2008).
In a 2020 review published in the journal Cancers (Basel), authors Rindi et al. noted that median overall survival in patients with metastatic well-differentiated PNETs ranged from 24 to 75 months. These differences were influenced by factors such as Ki-67 index, tumor burden, and access to treatments like PRRT and targeted therapy.
Surgical resection, even in the presence of metastasis, has shown improved outcomes in multiple retrospective analyses. For example, a study published in Annals of Surgery (Mazzaferro et al., 2016) found improved survival among patients who underwent hepatic resection for liver metastases from PNETs.
Systemic treatments have also improved disease control. Everolimus and sunitinib both demonstrated improved progression-free survival in randomized controlled trials (RADIANT-3 trial by Yao et al., New England Journal of Medicine, 2011; and Raymond et al., NEJM, 2011). PRRT also showed survival benefit in the NETTER-1 trial (Strosberg et al., New England Journal of Medicine, 2017).
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
What are pancreatic neuroendocrine tumors (PNETs)?
PNETs are rare tumors that develop in the hormone-producing cells of the pancreas, known as islet cells. They can be either benign or malignant and may produce excess hormones, leading to various symptoms.
What causes PNETs?
The exact cause is often unknown, but certain genetic syndromes, such as Multiple Endocrine Neoplasia type 1 (MEN1), can increase the risk of developing PNETs.
What are the symptoms of PNETs?
Symptoms vary depending on whether the tumor is functioning (producing hormones) or non-functioning. Functioning tumors may cause symptoms related to hormone overproduction, while non-functioning tumors might not cause noticeable symptoms until they grow larger.
How are PNETs diagnosed?
Diagnosis typically involves a combination of imaging tests (like CT or MRI scans), blood tests to measure hormone levels, and biopsy procedures to examine tumor cells.
What treatment options are available for PNETs?
Treatment depends on the tumor’s type, size, location, and whether it has spread. Options may include surgery, targeted drug therapy, hormone therapy, chemotherapy, and radiation therapy.
What is the prognosis for someone with a PNET?
Prognosis varies based on factors like tumor grade, stage at diagnosis, and overall health. Early-stage, well-differentiated tumors generally have a better outlook than advanced or poorly differentiated ones.
Are PNETs the same as typical pancreatic cancer?
No, PNETs are different from the more common pancreatic adenocarcinomas. They originate from different cell types and often have different treatment approaches and prognoses.
Can PNETs be prevented?
There are no specific preventive measures for PNETs, especially since their exact cause is often unclear. However, managing risk factors and undergoing regular check-ups may aid in early detection.
Do PNETs metastasize?
Yes, PNETs can metastasize, commonly to the liver and lymph nodes. The likelihood depends on the tumor’s characteristics and stage at diagnosis.
Is ongoing monitoring necessary after treatment?
Yes, regular follow-up appointments are crucial to monitor for potential recurrence and manage any long-term effects of treatment.