Natera has announced positive topline results from the phase III IMvigor011 trial in muscle-invasive bladder cancer (MIBC), highlighting the role of circulating tumor DNA (ctDNA) in guiding adjuvant immunotherapy. The study tested whether ctDNA, as a marker of molecular residual disease (MRD), could predict which patients benefit most from treatment with atezolizumab (Tecentriq®) following radical cystectomy.
The current standard of care for MIBC is radical cystectomy with or without neoadjuvant cisplatin-based chemotherapy. Yet relapse rates remain high, and up to half of patients recur within two years of surgery, and long-term survival is limited. This underscores the urgent need to identify high-risk patients early and personalize adjuvant therapy.
The IMvigor011 trial is the first phase III study in bladder cancer to prospectively evaluate a ctDNA-guided adjuvant immunotherapy strategy. Using Natera’s Signatera™ assay, the trial demonstrated that patients who were ctDNA-positive after surgery and treated with atezolizumab experienced statistically significant and clinically meaningful improvements in both disease-free survival (DFS) and overall survival (OS) compared with placebo.
IMvigor011 Trial Design and Population
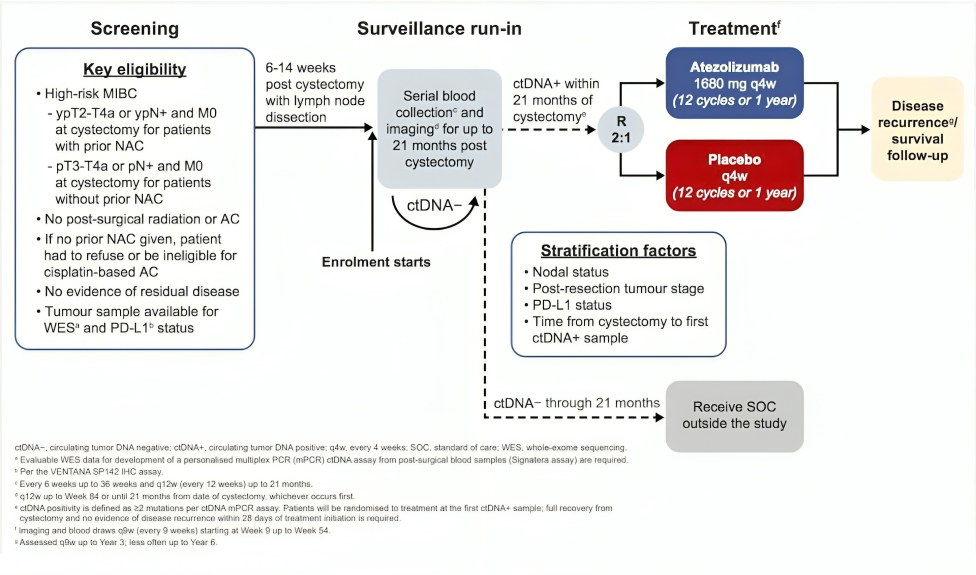
The IMvigor011 trial (NCT04660344) is a global, randomized, double-blind, placebo-controlled phase III study to test whether ctDNA can guide the use of adjuvant atezolizumab in muscle-invasive bladder cancer (MIBC). The study enrolled 761 patients who had undergone radical cystectomy with curative intent but remained at high risk for recurrence.
Following surgery, all patients entered a surveillance phase, during which blood samples were collected regularly and analyzed using Natera’s Signatera™ ctDNA assay for up to 21 months. Based on ctDNA results, patients were assigned to different treatment paths:
- ctDNA-positive patients (within 20 weeks post-cystectomy, with no radiographic evidence of disease) were randomized in a 2:1 ratio to receive either:
- Atezolizumab (Tecentriq®) at 1680 mg intravenously every 4 weeks, for up to 12 cycles (1 year), or
- Placebo on the same schedule.
- ctDNA-negative patients were not given adjuvant therapy but continued in surveillance, monitored closely with both imaging and ongoing ctDNA testing. Patients who remained ctDNA-negative at 12 months were transitioned to long-term follow-up.
The primary endpoint of the trial is disease-free survival (DFS) in ctDNA-positive patients, while secondary endpoints include overall survival (OS), ctDNA clearance rates, distant metastasis-free survival, and health-related quality of life.
Source: Dr. Tom Powles on X
Key Findings
The IMvigor011 trial demonstrated that treatment decisions guided by ctDNA status can meaningfully impact outcomes in muscle-invasive bladder cancer.
Among ctDNA-positive patients, adjuvant therapy with atezolizumab (Tecentriq®) led to a statistically significant and clinically meaningful improvement in both disease-free survival (DFS) and overall survival (OS) when compared with placebo. This confirms that the presence of molecular residual disease detected by ctDNA can serve as a strong predictor of who will benefit from checkpoint inhibition after cystectomy.
In contrast, ctDNA-negative patients had excellent outcomes without adjuvant therapy. A preliminary analysis presented earlier at the 2024 European Association of Urology (EAU) Annual Congress in Paris, France (April 5–8, 2024) showed that 171 patients who consistently tested negative during surveillance achieved overall survival rates of 100% at 12 months and 98% at 18 months, while disease-free survival was 92% at 12 months and 88% at 18 months. These results suggest that patients who remain ctDNA-negative can be safely spared adjuvant immunotherapy while maintaining favorable outcomes.
Professor Thomas Powles (Barts Cancer Institute, QMUL), lead investigator of IMvigor011, emphasized:
“In the future we won’t rely on pathology at surgery—or luck—to select patients for adjuvant treatment. Instead, identification of residual disease by ctDNA or other biomarkers will guide therapy. These data are a step towards that goal.”

What People Are Sharing on Social Media?
Yüksel Ürün, Medical Oncologist, Prof.Dr. at Ankara University — shared on X:
“ctDNA-guided adjuvant therapy finally delivering OS benefit in UC. A long road but worth it; this feels like a real step into the future of precision oncology in bladder cancer.”
Ashish M. Kamat, MD, MBBS, Endowed Professor, Surgeon, Former Fellowship Director — shared on X:
“A massive congratulations to Tom Powles and the entire team on the incredible results of the atezolizumab vs. placebo trial in ctDNA-positive bladder cancer. This study further solidifies the paradigm of using ctDNA as a dynamic biomarker to guide treatment decisions”
Future Implications
The IMvigor011 results support ctDNA as a biomarker-driven approach for guiding adjuvant immunotherapy in bladder cancer.
For the first time in a phase III setting:
- Patients with molecular residual disease (ctDNA-positive) clearly benefited from adjuvant atezolizumab.
- Patients who were ctDNA-negative did well with surveillance alone, avoiding unnecessary exposure to immunotherapy.
Natera has announced plans to submit a premarket approval application to the U.S. FDA to establish Signatera™ as a companion diagnostic for selecting MIBC patients for adjuvant atezolizumab. The IMvigor011 trial can be a breakthrough in uro-oncology: a ctDNA-guided, personalized approach that tailors adjuvant immunotherapy to patients who truly need it. You Can Read More About Immunotherapy for Bladder Cancer on OncoDaily
Read the Official Press Release by Natera