On March 26, 2025, the FDA approved cabozantinib (Cabometyx) for adult and pediatric patients (≥12 years) with previously treated, unresectable, locally advanced, or metastatic well-differentiated pancreatic neuroendocrine tumors (pNET) and extra-pancreatic neuroendocrine tumors (epNET).
What are pancreatic and extra pancreatic neuroendocrine tumors?
Pancreatic neuroendocrine tumors (pNETs) are a group of tumors that arise from islet cells in the pancreas. While they may look similar to carcinoid tumors of the gastrointestinal tract, they have distinct biological behavior and treatment responses, requiring a different approach to management.
Most pNETs occur sporadically, but some develop as part of Multiple Endocrine Neoplasia type 1 (MEN1), a hereditary syndrome caused by mutations in the MEN1 tumor suppressor gene (chromosome 11q13). MEN1 is associated with tumors in the pituitary, parathyroid, and pancreas, often leading to multiple pancreatic tumors in affected individuals.
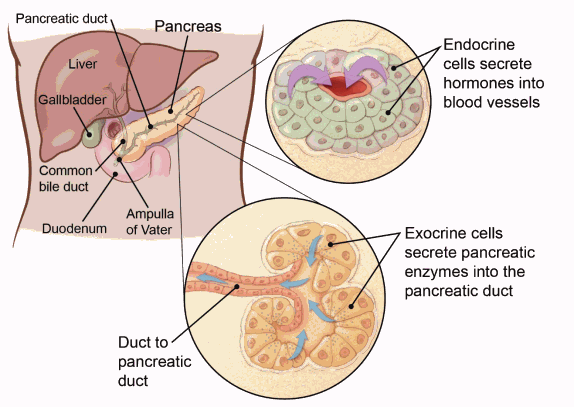
pNETs are classified as functional or nonfunctional:
Functional pNETs produce excess hormones, leading to specific clinical syndromes, mot common functional NETs are gastrinomas, insulinomas, glucagonomas, somatostatinomas and VIPomas
- Gastrinoma – excess gastrin, causing severe acid production and ulcers.
- Insulinoma – excess insulin, leading to recurrent hypoglycemia.
- Glucagonoma – excess glucagon, causing diabetes and skin rash.
- Somatostatinoma – excess somatostatin, disrupting multiple hormone pathways.
- VIPoma – excess vasoactive intestinal peptide (VIP), leading to profuse diarrhea and electrolyte imbalance.
Nonfunctional pNETs do not secrete significant hormones but can grow aggressively and metastasize. Due to the lack of early symptoms, they are often diagnosed at an advanced stage.
Extra-pancreatic neuroendocrine tumors (epNETs) are well-differentiated neuroendocrine tumors (NETs) that develop outside the pancreas. Like pancreatic NETs (pNETs), they originate from neuroendocrine cells, which produce hormones and are found throughout the body. epNETs can occur in various organs, most commonly in the gastrointestinal (GI) tract and lungs.
What drug is cabozantinib and mechanism of action
Cabozantinib is an oral inhibitor that targets multiple tyrosine kinase receptors, including VEGFR2, c-MET, and RET. By inhibiting VEGFR and c-MET, it reduces resistance to VEGFR inhibitors through the c-MET pathway. In patients with progressive metastatic medullary thyroid cancer (MTC), cabozantinib significantly improved progression-free survival (PFS), with a median of 11.2 months compared to 4 months in the placebo group (P<0.001), across all patient subgroups, including those with or without prior ITK treatments and different RET mutation statuses.
In patients with advanced renal cell carcinoma (RCC) who had progressed after previous VEGFR ITK treatment, cabozantinib also showed a notable increase in overall survival (OS), with a median of 21.4 months compared to 16.5 months in the everolimus group (P<0.0003). Cabozantinib has been approved for the treatment of progressive metastatic MTC and advanced RCC. It offers a new treatment option for MTC in clinical trials (without reimbursement in this indication) and advanced RCC (administered in hospitals). Currently cabozantinib has been approved for pancreatic and extrapancreatic NETs, for patients who had progressed on previous treatment.
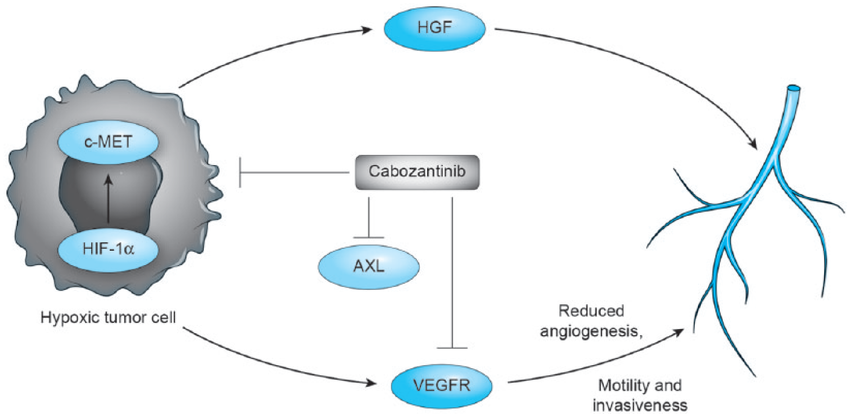
The drug’s side effects are similar to those of other anti-angiogenic therapies, and its management requires careful monitoring of secondary effects.
The CABINET trial and its impact
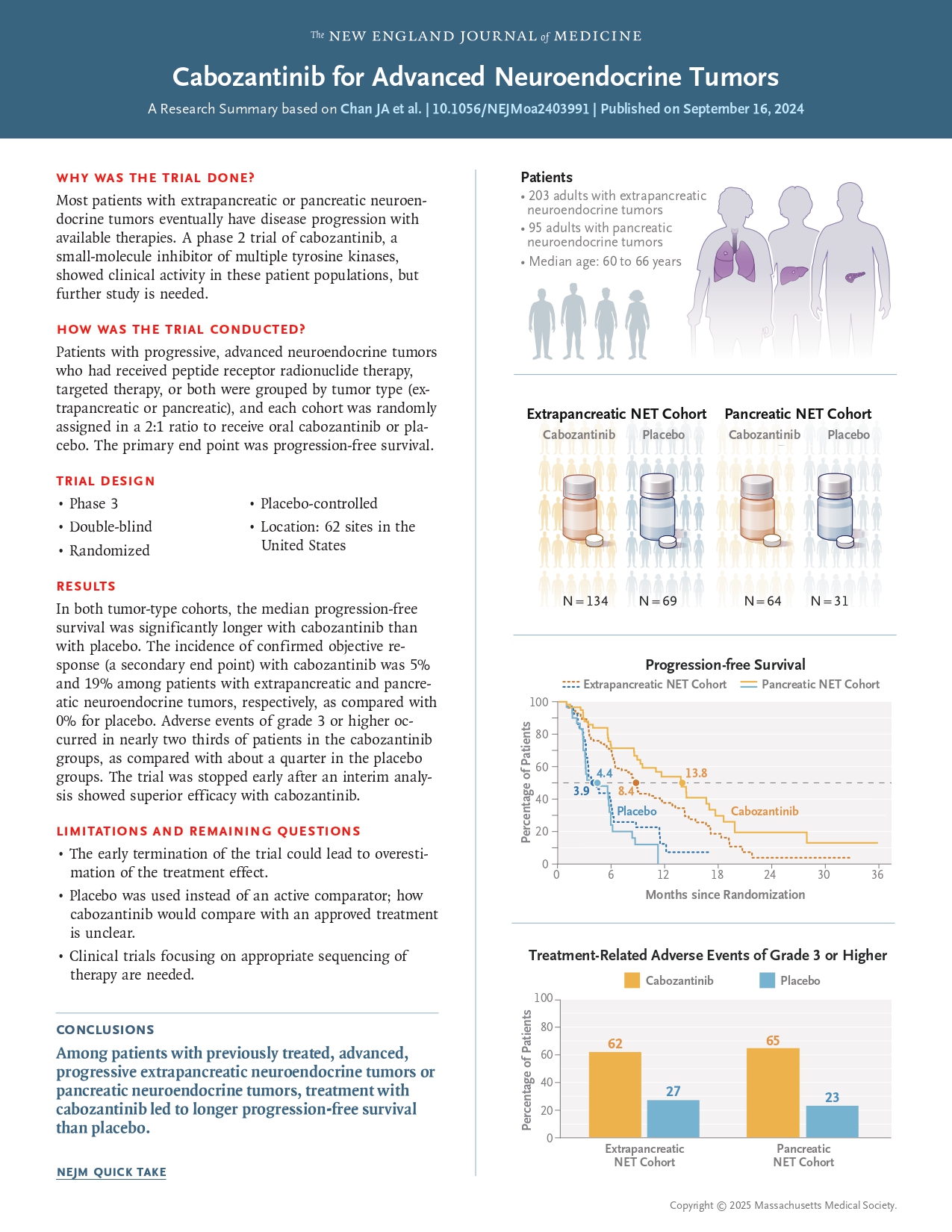
CABINET trial was a randomized, placebo-controlled, double-blind Phase 3 trial assessing the efficacy and safety of cabozantinib in patients with advanced pNETs and epNETs. Eligible patients had histologically confirmed, progressive, well-differentiated NETs despite prior systemic therapy. Participants were stratified based on primary tumor location and other clinical factors.
Study Design
The trial enrolled 298 patients in total, divided into two cohorts:
- pNET cohort: 99 patients
- epNET cohort: 199 patients
Patients were assigned in a 2:1 ratio to receive either:
- Cabozantinib 60 mg once daily
- Placebo
Primary Endpoint was Progression-free survival (PFS), assessed by an independent radiology review committee per RECIST 1.1 criteria. Secondary Endpoints were Overall response rate (ORR) and overall survival (OS).
Results
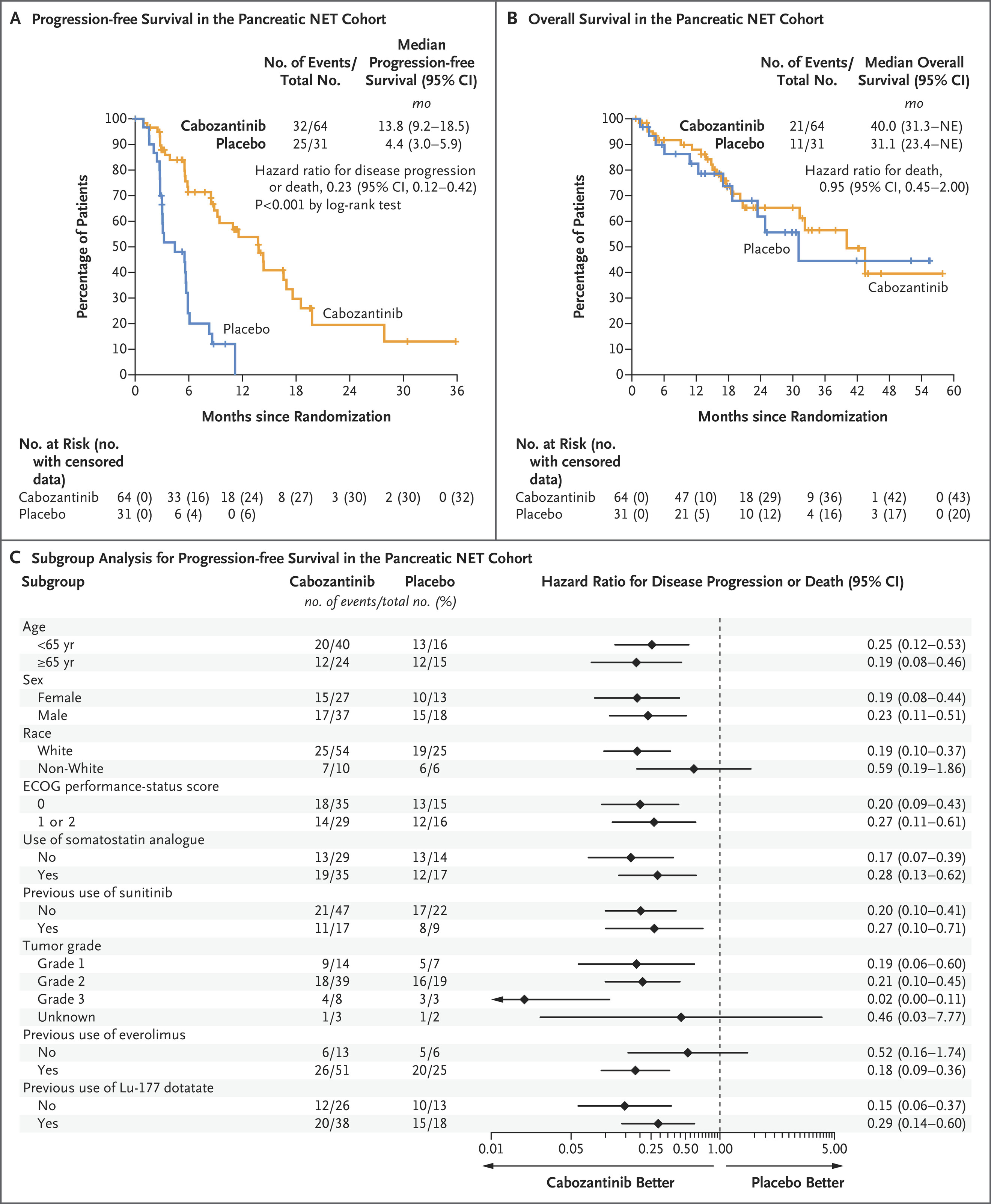
Cabozantinib demonstrated a statistically significant and clinically meaningful improvement in PFS for patients with advanced, well-differentiated NETs, including both pancreatic and extra-pancreatic subtypes. The results establish cabozantinib as an effective treatment option for patients with progressive NETs following prior therapy. Further research is needed to clarify overall survival benefits and optimize patient selection to balance efficacy and toxicity. This study highlights the growing role of targeted therapy in the management of neuroendocrine tumors.
- pNET Cohort:
- Median PFS: 13.8 months (95% CI: 8.9–17.0) with cabozantinib vs. 3.3 months (95% CI: 2.8–5.7) with placebo (HR: 0.22, p<0.0001).
- Objective Response Rate (ORR): 18% (95% CI: 10–30) in cabozantinib arm vs. 0% (95% CI: 0–11) in placebo arm.
- Overall Survival (OS): Data were immature, with 48% of patients in the cabozantinib group and 52% in the placebo group deceased at the time of analysis (HR: 1.01).
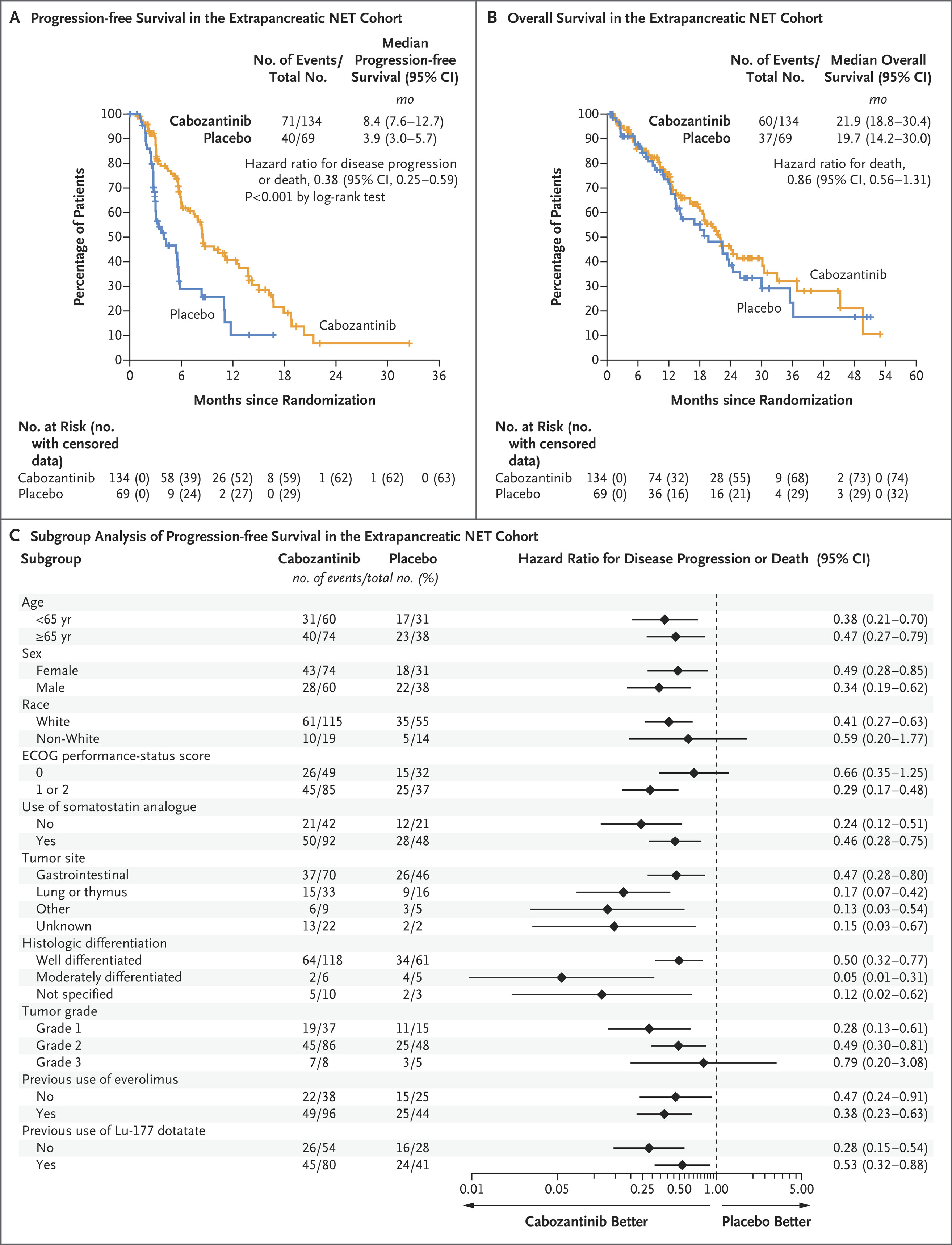
- epNET Cohort:
- Median PFS: 8.5 months (95% CI: 6.8–12.5) with cabozantinib vs. 4.2 months (95% CI: 3.0–5.7) with placebo (HR: 0.40, p<0.0001).
- ORR: 5% (95% CI: 2.2–11) with cabozantinib vs. 0% (95% CI: 0–5) with placebo.
- OS: Data were immature, with 63% of cabozantinib-treated patients and 60% of placebo-treated patients deceased at analysis (HR: 1.05).
Key Findings
- Cabozantinib significantly prolonged PFS in both pNET and epNET cohorts compared to placebo, with hazard ratios of 0.22 and 0.40, respectively.
- ORR was higher in the cabozantinib group compared to placebo, particularly in pNETs (18% vs. 0%).
- OS results were inconclusive due to the high crossover rate in the placebo group (52% in pNETs and 37% in epNETs).
- The safety profile was consistent with previous cabozantinib studies. Grade 3 or higher adverse events occurred in 62–65% of patients receiving cabozantinib, compared to 23–27% in the placebo group.
On March 26, 2025, the FDA approved cabozantinib (Cabometyx) for the treatment of adults and pediatric patients (12 years and older) with unresectable, locally advanced, or metastatic, well-differentiated pancreatic neuroendocrine tumors (pNET) and extra-pancreatic neuroendocrine tumors (epNET) who have previously been treated.Cabozantinib significantly improved PFS in both pNET and epNET groups compared to placebo.
The drug showed an ORR of 18% in pNET patients, with a much lower response in the placebo group. Survival data is still being evaluated, as many patients in the placebo group crossed over to receive cabozantinib.
More information here
Written by Sona Karamyan, MD


