Sorafenib is a targeted oral cancer therapy that works by disrupting the growth signals cancer cells rely on to survive and spread. By blocking multiple proteins and cutting off the blood supply tumors need, sorafenib slows cancer progression on several fronts. As a multikinase inhibitor, it interferes with key cellular pathways that drive tumor growth and angiogenesis. In this article, we explore how sorafenib works, its common side effects, recommended dosage, and what patients can expect during treatment.
Which company produced Sorafenib?
Sorafenib, marketed under the brand name Nexavar, was developed and is marketed by Bayer, a global pharmaceutical and life sciences company based in Germany. Founded in 1863, Bayer is one of the oldest and most established companies in the healthcare industry. It focuses on innovation in pharmaceuticals, consumer health, and agricultural solutions. In oncology, Bayer has developed several targeted therapies and is committed to advancing treatment options for various types of cancer through research and global collaborations.
How does Sorafenib work?
Nexavar is a targeted cancer therapy known as a multikinase inhibitor. It works by blocking several proteins (called kinases) that cancer cells use to grow, divide, and develop new blood vessels. By interfering with these key pathways, sorafenib helps slow the growth and spread of cancer.
Sorafenib targets multiple signaling pathways, including:
- RAF kinases (CRAF and BRAF): These enzymes are part of the MAPK/ERK pathway, which controls cell growth and survival. Blocking RAF kinases helps stop cancer cells from multiplying.
- VEGFR (vascular endothelial growth factor receptors): These receptors promote the growth of blood vessels that feed tumors. Inhibiting VEGFR helps cut off the tumor’s blood supply, limiting its growth.
- PDGFR (platelet-derived growth factor receptors): These are involved in cell growth and the stability of blood vessels. Blocking PDGFR adds another layer of anti-tumor activity.
By targeting both cancer cells and the blood vessels that support them, Nexavar slows tumor progression and may help stabilize or reduce tumor size over time. This multi-targeted approach makes it effective in treating certain advanced cancers.
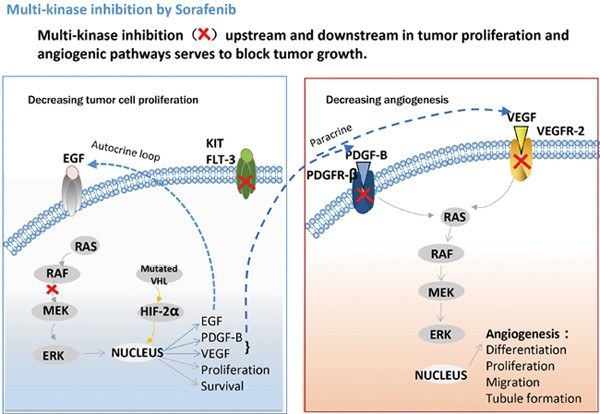
Cellular targets of sorafenib. Sorafenib blocks receptor tyrosine kinase signaling (VEGFR, PDGFR, c-Kit and RET) and inhibits downstream Raf serine/threonine kinase activity to prevent tumor growth by anti-angiogenic, antiproliferative and/or pro-apoptotic effects (from Bayer website).
What Cancers Is Sorafenib Approved to Treat?
Nexavar is approved for the treatment of several advanced cancers, offering targeted therapy to patients with difficult-to-treat conditions. Sorafenib is approved for advanced renal cell carcinoma, with FDA approval granted on December 20, 2005. It was the first oral targeted therapy approved for advanced renal cell carcinoma (RCC), a type of kidney cancer that has spread or cannot be surgically removed.
Sorafenib is also approved for unresectable hepatocellular carcinoma, with FDA approval granted on November 19, 2007. It is used for patients with hepatocellular carcinoma (HCC), a form of liver cancer that cannot be treated with surgery.
Additionally, Nexavar is approved for metastatic differentiated thyroid cancer (DTC), with FDA approval granted on November 22, 2013. This approval was specifically for treating metastatic differentiated thyroid cancer that is resistant to radioactive iodine therapy.
Learn more about Thyroid Cancer: Symptoms, Causes, Stages, Diagnosis on OncoDaily.
What research is behind the approval?
Nexavar has been extensively studied in clinical trials to evaluate its role in treating advanced cancers.
Sorafenib for Renal Cell Carcinoma
The approval of Nexavar for advanced renal cell carcinoma was supported by results from a phase 3, randomized, double-blind, placebo-controlled trial published in The New England Journal of Medicine in 2007. The study included 903 patients with advanced clear-cell renal cell carcinoma that had progressed after standard therapy. Patients received either sorafenib (400 mg twice daily) or placebo.
The trial showed that sorafenib significantly improved progression-free survival, with a median of 5.5 months compared to 2.8 months in the placebo group (hazard ratio: 0.44; P<0.01). Although early data suggested a reduction in the risk of death, the overall survival benefit did not meet the pre-specified threshold for statistical significance.
Common side effects included diarrhea, rash, fatigue, and hand–foot skin reactions, while hypertension and cardiac ischemia were less frequent but more serious. Overall, sorafenib demonstrated clinical benefit in delaying disease progression in advanced kidney cancer, though with manageable side effects.

Sorafenib for Hepatocellular Carcinoma
Key evidence for sorafenib in advanced liver cancer comes from a large phase 3 trial published in The New England Journal of Medicine in 2008. This multicenter, double-blind, placebo-controlled study involved 602 patients with advanced hepatocellular carcinoma (HCC) who had not received prior systemic therapy.
Patients were randomly assigned to receive either sorafenib (400 mg twice daily) or placebo. The main goals were to assess overall survival and time to symptomatic progression, with secondary outcomes including time to radiologic progression and treatment safety.
At the second interim analysis, the trial was stopped due to clear benefit. Sorafenib improved median overall survival to 10.7 months versus 7.9 months with placebo (HR 0.69; P<0.001). While there was no significant difference in time to symptom progression, radiologic progression was delayed (5.5 vs. 2.8 months; P<0.001). Partial tumor responses were seen in 2% of sorafenib-treated patients compared to 1% with placebo.
Common side effects in the sorafenib group included diarrhea, weight loss, hand–foot skin reaction, and low phosphate levels. These results demonstrated that sorafenib offers a meaningful survival benefit and delays disease progression in patients with advanced HCC.

Sorafenib for Thyroid cancer
Strong clinical evidence for sorafenib in differentiated thyroid cancer came from the DECISION trial, a phase 3, randomized, double-blind, placebo-controlled study published in The Lancet Oncology in 2014. The trial involved 417 adults with locally advanced or metastatic differentiated thyroid cancer that was no longer responsive to radioactive iodine and had progressed within the last 14 months.
Participants were randomly assigned to receive either sorafenib (400 mg twice daily) or placebo. The primary endpoint was progression-free survival (PFS), assessed every 8 weeks by independent review. Tumor tissue was also tested for BRAF and RAS mutations, and serum thyroglobulin levels were monitored throughout.
Results showed that sorafenib significantly prolonged median progression-free survival to 10.8 months versus 5.8 months with placebo (hazard ratio 0.59; p<0.0001). This benefit was consistent across all clinical and genetic subgroups, regardless of BRAF or RAS mutation status. Nearly all patients in the sorafenib group (98.6%) experienced some side effects, most of which were mild to moderate (grade 1 or 2). The most common adverse events included hand–foot skin reaction (76.3%), diarrhea (68.6%), alopecia (67.1%), and rash or desquamation (50.2%).
The DECISION trial confirmed that Nexavar offers a meaningful delay in disease progression for patients with advanced, radioactive iodine-refractory thyroid cancer, with a safety profile consistent with previous findings.
60421-9/asset/696e8d51-25f8-4334-bae6-f7f271a50f23/main.assets/gr3_lrg.jpg)
Sorafenib side effects and its management
Nexavar is a multikinase inhibitor used in the treatment of various cancers. While it offers therapeutic benefits, it is associated with a range of side effects. Understanding these adverse effects and their management is crucial for optimizing patient outcomes.
Common Side Effects
Patients commonly experience side effects such as hand-foot skin reaction, diarrhea, fatigue, skin rash, hair thinning, and high blood pressure. Hand-foot skin reaction typically presents as redness, pain, or blistering on the palms and soles, which can interfere with daily activities. Diarrhea is often reported and may become problematic if left unmanaged. Fatigue is another frequent issue, which can persist throughout the course of therapy. Skin-related effects, such as rash and dryness, are common and usually mild to moderate in severity. Hair thinning may occur gradually. Elevated blood pressure is also observed, requiring routine monitoring and, when necessary, antihypertensive therapy.
Less common and serious side effects
Less frequently, patients may experience nausea, vomiting, loss of appetite, weight loss, constipation, and joint pain. These effects may not be as widespread but can still significantly impact a patient’s well-being. Gastrointestinal discomfort can interfere with nutrition, while joint pain may reduce physical activity and mobility.
Serious side effects are rare but require immediate attention. These include severe skin reactions such as Stevens-Johnson syndrome, liver dysfunction indicated by elevated liver enzymes or jaundice, abnormal bleeding, heart problems such as chest pain or arrhythmia, and gastrointestinal perforation, which causes severe abdominal pain. These adverse events, while uncommon, can be life-threatening and should be reported to a healthcare provider without delay.
Management of side effects
Effective management of sorafenib’s side effects involves dose interruptions or reductions, supportive medications, and symptom-focused interventions. Hand-foot skin reaction can be managed with moisturizers, topical treatments, and pressure avoidance. Diarrhea may require anti-diarrheal medications and hydration. High blood pressure can be controlled with standard antihypertensives. Fatigue and appetite loss may be addressed through rest, nutritional support, and energy conservation strategies. Close monitoring through regular blood work and follow-up visits is essential for early detection and intervention.
What is the Recommended Dosage of Sorafenib?
Nexavar is a multikinase inhibitor used to treat advanced cancers like renal cell carcinoma, hepatocellular carcinoma, and differentiated thyroid cancer. The typical dosage is 400 mg twice daily, but adjustments may be necessary based on side effects, especially dermatologic toxicity.
Dosage modifications are common if patients experience skin reactions such as rashes or hand-foot skin syndrome. For renal cell carcinoma and hepatocellular carcinoma, if these reactions occur, the dosage may be reduced or the frequency decreased to every other day. For thyroid cancer, the dose can be reduced to 600 mg daily or further adjusted to 200 mg depending on the severity of side effects.
In patients with renal or hepatic impairment, sorafenib does not require dose adjustments for mild-to-moderate conditions, but caution is advised in severe cases due to limited safety data.
Proper management of side effects, including monitoring for skin reactions and adjusting the dose as needed, helps ensure sorafenib’s effectiveness while minimizing discomfort.
Learn more about Kidney Cancer: Symptoms ,Causes, Stages, Diagnosis and Treatment on OncoDaily.
How is Sorafenib administered?
Nexavar is administered orally, and it is recommended to take the medication one hour before or two hours after meals to optimize absorption. It is important to regularly monitor the patient’s blood pressure (BP) during treatment, as sorafenib can potentially cause hypertension, which requires management.
What to Avoid During Sorafenib Treatment?
During sorafenib treatment, it’s important to avoid certain substances and activities to minimize side effects. Grapefruit and grapefruit juice should be avoided because they can interfere with the metabolism of Nexavar, increasing the risk of side effects. Strong CYP3A4 inhibitors, such as ketoconazole, may also increase sorafenib levels and require careful monitoring. Patients should also avoid excessive sun exposure and wearing tight clothing during skin toxicity, as Nexavar can cause skin reactions like rashes and hand-foot syndrome. Additionally, sorafenib is not recommended during pregnancy or breastfeeding, and live vaccines should be avoided due to potential immune suppression. Alcohol intake should be limited to prevent liver toxicity, especially in patients with liver conditions like hepatocellular carcinoma (HCC).
Sorafenib effectiveness over time
A 2023 study published in PubMed (PMID: 37969411) evaluated the real-world effectiveness of sorafenib as first-line therapy for unresectable hepatocellular carcinoma (uHCC) at two U.S. centers. In this retrospective analysis of 134 patients treated between 2016 and 2019, the median progression-free survival was 2.9 months and overall survival was 8.5 months. Most patients experienced disease progression or death during treatment, with 30% discontinuing due to toxicity. These findings highlight the need to consider newer treatment options that may offer improved outcomes over Nexavar.
Sorafenib 2025: Latest Updates and Breakthroughs in Cancer Treatment
Published in ECANCER (March 13, 2025), the SORA-COG pilot study explored the neurocognitive effects of Nexavar in desmoid tumor (DT) patients. Among 50 participants—those on sorafenib, treatment-naïve patients, and healthy controls—no significant cognitive impairments were found. However, patients on long-term sorafenib showed higher anxiety levels, suggesting a need for routine mental health support during treatment.

Read more about New Paper Alert: A pilot study on the neurocognitive effect of sorafenib on patients with desmoid tumors on OncoDaily.
At the ESMO Sarcoma and Rare Cancers Congress 2025, a study on sorafenib and everolimus for treating refractory osteosarcoma and Ewing’s sarcoma in children was presented. Despite chemotherapy, progression-free survival (PFS) typically drops to zero after 6-8 months.
From May 2018 to August 2023, 12 patients (average age 13.4 years) received sorafenib (150-200 mg/m²) and everolimus (2.5-5 mg/m²) for 28-day cycles. Treatment continued until disease progression or severe toxicity. Results showed skin erythema (100%), palm-podontal syndrome (9%), and oral mucositis (18%) as common side effects. Four patients had partial response, and eight had disease stabilization. The one-year PFS was 36.2%, and six-month PFS was 54%, with the longest PFS of 13.3 months.
In conclusion, the combination of sorafenib and everolimus showed encouraging efficacy and tolerability, but further research is needed.
Ongoing trials with Sorafenib
A Phase 2 clinical trial (NCT06227221) is evaluating whether sorafenib can help preserve beta cell function in adults with newly diagnosed type 1 diabetes (T1DM). Unlike insulin, which only controls blood sugar, sorafenib may address the autoimmune cause of T1DM by reducing inflammation. Previous research in mice showed that sorafenib lowered harmful immune responses and protected pancreatic cells. Already approved for certain cancers, sorafenib is now being tested over a 26-week period to see if it can slow disease progression in humans with new-onset T1DM.
Written by Mariam Khachatryan, MD
FAQ
What is Sorafenib used for?
Sorafenib is an oral targeted therapy approved to treat several types of cancer, including liver cancer (hepatocellular carcinoma), kidney cancer (advanced renal cell carcinoma), and thyroid cancer (differentiated thyroid carcinoma that is refractory to radioactive iodine treatment).
How does Nexavar work?
Nexavar works by blocking multiple proteins (kinases) involved in tumor cell growth and angiogenesis (the formation of new blood vessels that supply tumors). It targets RAF kinases, VEGFR, PDGFR, and other pathways to inhibit cancer progression.
Is Sorafenib a chemotherapy drug?
No, Sorafenib is not classified as chemotherapy. It is a targeted therapy, which means it specifically interferes with cancer cell pathways rather than killing rapidly dividing cells indiscriminately like traditional chemotherapy.
How is Nexavar taken?
Nexavar is taken orally, usually as a tablet twice daily (every 12 hours) without food or with a low-fat meal. It's important to take it at the same time each day for consistent blood levels.
What is the recommended dosage of Sorafenib?
The standard recommended dose for adults is 400 mg twice daily, but your doctor may adjust the dose based on how well you tolerate the treatment or any side effects that occur.
What are the common side effects of Nexavar?
Common side effects include diarrhea, fatigue, hand-foot skin reaction (HFSR), loss of appetite, rash, and high blood pressure.
What should I avoid while taking Nexavar?
While on SNexavar, avoid grapefruit, as it can interfere with how the drug is metabolized. Also, avoid excessive sun exposure, as Sorafenib may increase skin sensitivity.


