The ESMO 2025 Congress will deliver major updates in gastroesophageal junction (GEJ), esophageal and gastric cancers. These studies span perioperative, metastatic, and locally advanced settings, featuring immune checkpoint inhibitors, bispecific antibodies, and novel targeted approaches. This overview highlights the most clinically relevant esophageal and gastric cancer trials to be presented at ESMO 2025, with potential to inform practice and shape future standards of care.
LEAP-014 Trial: Lenvatinib + Pembrolizumab + Chemotherapy in Untreated Metastatic ESCC
Abstract: LBA79
Presenter: Jong-Mu Sun (Seoul, Republic of Korea)
Trial Type: Phase III
Session: Proffered Paper session
LEAP-014 is a randomized phase 3 study evaluating lenvatinib plus pembrolizumab and chemotherapy versus pembrolizumab plus chemotherapy in untreated metastatic esophageal squamous cell carcinoma (ESCC). The trial aims to determine whether VEGF/FGF pathway inhibition can further enhance response and survival outcomes when added to first-line chemoimmunotherapy in ESCC.
Why it matters: This could establish a new triplet backbone by adding VEGF/FGF pathway inhibition to first-line chemoimmunotherapy, potentially deepening responses and prolonging survival in this historically hard-to-treat disease.
INTEGRATE IIb Trial: Regorafenib Plus Nivolumab vs Chemotherapy in Previously Treated G/GEJ Cancer
Abstract: LBA80
Presenter: David Goldstein (Sydney, Australia)
Trial Type: Phase III (AGITG Intergroup [NHMRC-CTC/IKF/AIO, ACCRU, TCOG/NHRI])
Session: Proffered Paper session
INTEGRATE IIb is a randomized phase 3 trial comparing regorafenib plus nivolumab against investigator’s choice of chemotherapy in patients with previously treated gastric or GEJ cancer. This combination seeks to exploit potential synergy between multikinase inhibition and PD-1 blockade to improve outcomes over standard chemotherapy in the second- or third-line setting.
Read more about Opdivo (Nivolumab): Uses in Cancer, Side Effects, Dosages, Expectations on OncoDaily.
The results of the INTEGRATE IIa Phase III trial were published in Journal of Clinical Oncology in 2024 (Vol. 43, No. 4). This global, double-blind, placebo-controlled study evaluated regorafenib plus best supportive care versus placebo in patients with refractory advanced gastric or gastroesophageal junction cancer who had progressed after at least two prior lines of therapy.
Among 251 participants, regorafenib significantly improved overall survival compared with placebo (HR = 0.68; 95% CI, 0.52–0.90; P = .006), with a median OS of 4.5 months versus 4.0 months and 12-month survival rates of 19% versus 6%. The pooled analysis of INTEGRATE I and IIa confirmed this benefit (HR = 0.70; P = .001). Regorafenib also improved progression-free survival (HR = 0.53; P < .0001) and delayed deterioration in global quality of life. The safety profile was consistent with earlier studies.
Why it matters: Building on OS and PFS gains seen in INTEGRATE I and IIa, this trial could validate VEGF blockade + immunotherapy as a viable late-line regimen, offering a chemotherapy-free option for refractory disease.
MATTERHORN Trial: Durvalumab Plus FLOT in Resectable Gastric/GEJ Adenocarcinoma
Abstract: LBA81
Presenter: Josep Tabernero (Barcelona, Spain)
Trial Type: Phase III
Session: Proffered Paper session
MATTERHORN is a global randomized phase 3 trial testing perioperative durvalumab in combination with FLOT (5-FU, leucovorin, oxaliplatin, docetaxel) chemotherapy in patients with resectable gastric or GEJ adenocarcinoma. The presentation will report event-free survival (EFS) and pathological response outcomes, key indicators of whether perioperative PD-L1 inhibition can improve cure rates beyond chemotherapy alone.
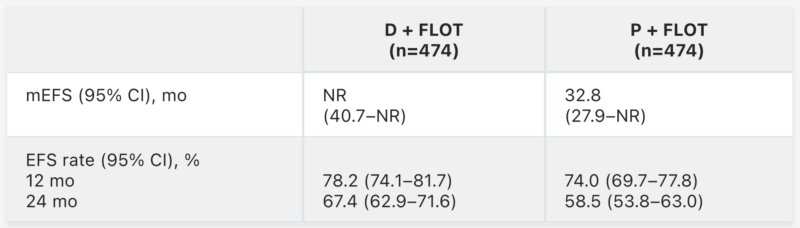
The previous results of the Phase 3 MATTERHORN trial were published in Journal of Clinical Oncology in 2025 (Vol. 43, Suppl 17). This global, randomized, double-blind study compared perioperative durvalumab plus FLOT (D + FLOT) with placebo plus FLOT in 948 patients with resectable, locally advanced gastric or gastroesophageal junction cancer.
At a median follow-up of 31.5 months, D + FLOT significantly improved event-free survival (HR 0.71; 95% CI 0.58–0.86; p<0.001), with median EFS not reached versus 32.8 months for placebo. Two-year EFS rates were higher with D + FLOT (67.4% vs 58.5%), and overall survival trended favorably (HR 0.78) with final OS data awaited. Durvalumab did not delay surgery or adjuvant therapy, and grade 3–4 adverse events were comparable between arms. These results support D + FLOT as a potential new standard of care for resectable gastric and GEJ cancer.
Why it matters: Early results already demonstrated a significant EFS improvement; this update could cement D+FLOT as a new standard perioperative regimen, shifting practice toward immunotherapy-based curative strategies.
KC-WISE: KN026 Plus Chemotherapy in Previously Treated HER2+ GC/GEJC
Abstract: LBA78
Presenter: Jianming Xu (Beijing, China)
Trial Type: Phase II (Interim Analysis)
Session: Proffered Paper session
KN026 is a bispecific HER2-targeted antibody designed to enhance receptor engagement and overcome trastuzumab resistance. The KC-WISE trial evaluates KN026 plus chemotherapy in previously treated HER2-positive gastric or GEJ cancer. Interim data will provide insight into response rates, durability, and safety, informing the sequencing of HER2-targeted regimens.
Why it matters: This study could expand the treatment arsenal beyond trastuzumab, providing an effective option for patients who progress after first-line HER2-targeted therapy and helping define optimal sequencing in HER2+ disease.
PHERFLOT/IKF-053: Pembrolizumab + Trastuzumab + FLOT in Perioperative HER2+ EG Cancer
Abstract: 2095MO
Presenter: Joseph Tintelnot (Hamburg, Germany)
Trial Type: Phase II (Interim Analysis)
Session: Mini Oral session
PHERFLOT is a phase II AIO study testing perioperative pembrolizumab and trastuzumab combined with FLOT chemotherapy in HER2-positive localized esophagogastric adenocarcinoma. The interim analysis will evaluate pathological response and safety, addressing whether adding PD-1 inhibition to HER2-targeted perioperative therapy can deepen responses and improve long-term outcomes.
Why it matters: This trial explores a triple-modality approach (chemo + HER2 blockade + immunotherapy) in the curative setting, which could meaningfully raise pathological complete response rates and long-term survival.
EC-CRT-002: Tislelizumab + Induction Chemo + Concurrent CRT in Locally Advanced ESCC
Abstract: LBA82
Presenter: Mian Xi (Guangzhou, China)
Trial Type: Phase II
Session: Mini Oral session
EC-CRT-002 is a randomized multicenter phase II trial exploring tislelizumab combined with induction chemotherapy and concurrent chemoradiotherapy for locally advanced ESCC. This study investigates whether immune checkpoint blockade can improve complete response rates and long-term control compared with chemoradiation alone.
Why it matters: Positive data could establish a new immuno-CRT paradigm for ESCC, improving complete response rates and potentially reducing recurrence risk without compromising tolerability.
Read more about Tislelizumab (Tevimbra): Uses in Cancer, Side Effects, Dosage, Expectations on OncoDaily.
COMPASSION-15 Trial: Cadonilimab Plus Chemotherapy as First-Line Therapy
Abstract: 2098MO
Presenter: Lin Shen (Beijing, China)
Trial Type: Phase III
Session: Mini Oral session
COMPASSION-15 evaluates cadonilimab, a PD-1/CTLA-4 bispecific antibody, plus chemotherapy versus chemotherapy alone as first-line therapy for advanced gastric or GEJ adenocarcinoma. Final results will include PFS, OS, and safety, clarifying whether dual-checkpoint blockade upfront can set a new benchmark in this population.
Why it matters: This is the first phase 3 trial of a PD-1/CTLA-4 bispecific in GI cancer; if positive, it could redefine first-line therapy and offer a new benchmark for dual-checkpoint blockade combinations.
CLDN18.2-Targeted ADC RC118 + PD-1 Blockade or RC148 (PD-1/VEGF Bispecific)
Abstract: LBA83
Presenter: Yiyi Yu (Shanghai, China)
Trial Type: Early-phase combination study
Session: Mini Oral session
This early-phase study explores RC118, a CLDN18.2-directed ADC, combined with PD-1 blockade, and RC148, a PD-1/VEGF bispecific antibody, in advanced gastric/GEJ adenocarcinoma. ESMO 2025 data will provide first efficacy and safety signals, potentially informing future combination strategies for biomarker-selected patients.
Why it matters: Early signals could guide the future development of ADC + immunotherapy and bispecific regimens, paving the way for more personalized approaches in CLDN18.2-positive disease.
Takeaway
These eight ESMO 2025 presentations collectively span the full spectrum of esophageal and gastric cancer care — from perioperative intensification with immunotherapy and HER2-targeted combinations to novel bispecific antibodies, ADCs, and VEGF/PD-1 doublets in metastatic disease. Together, they highlight the field’s shift toward biomarker-driven, combination-based, and potentially curative strategies.

Read more about Top Colorectal Cancer Trials to Watch at ESMO 2025 on OncoDaily.


