The ASCO 2025 Plenary Session delivered pivotal results across a spectrum of cancers, with landmark findings from the ATOMIC, MATTERHORN, SERENA-6, VERIFY, and NIVOPOSTOP trials. These studies collectively signal a transformative shift in adjuvant and precision oncology strategies, spanning colorectal, gastric, breast, hematologic, and head and neck malignancies.
MATTERHORN Trial: Durvalumab + FLOT Improves EFS in Resectable Gastric/GEJ Cancer
MATTERHORN is Global, double-blind, phase 3 trial (N=948) in patients with resectable stage II–IVa gastric or GEJ adenocarcinoma. Randomized to perioperative FLOT ± durvalumab. Both arms received 4 cycles of FLOT pre/post surgery; durvalumab (or placebo) given every 4 weeks, with 10 additional maintenance doses post-therapy.
Primary Endpoint was Event-free survival (EFS).
Key Findings
- Median EFS: not reached (durvalumab arm) vs 32.8 months (placebo arm), HR 0.71; p<0.001
- 24-month EFS: 67.4% vs 58.5%
- Early OS data trending positive (HR 0.78)
Safety: Grade 3–4 AEs similar; no delays to surgery or adjuvant treatment.
Matterhorn trial presents the first evidence of perioperative immunotherapy benefit in resectable gastric/GEJ cancer. Supports global adoption of durvalumab + FLOT.
You can read the Full Article in NEJM.
ATOMIC Trial: Atezolizumab + mFOLFOX6 Sets New Standard in Stage III dMMR Colon Cancer
ATOMIS is Phase 3, randomized trial (N=712) comparing mFOLFOX6 chemotherapy with or without atezolizumab in resected stage III dMMR colon cancer. Patients received 12 cycles of mFOLFOX6 ± atezolizumab every 2 weeks; the atezolizumab group continued with 13 cycles of monotherapy.
Primary Endpoint is Disease-free survival (DFS).
Key Findings
- DFS at 3 years: 86.4% with atezolizumab vs 76.6% with mFOLFOX6 alone (HR 0.50; p<0.0001)
- Benefit consistent across subgroups including older adults and high-risk features
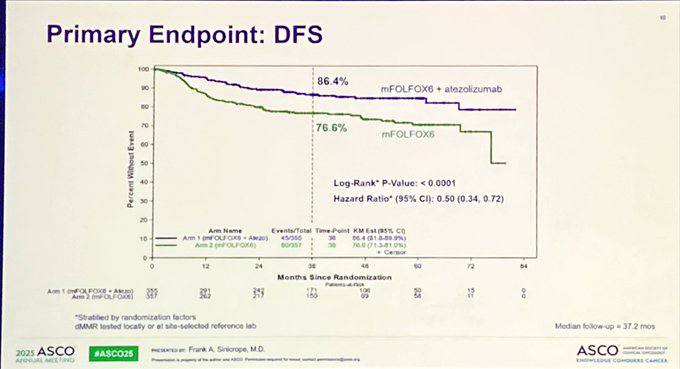
Safety: Grade ≥3 adverse events: 71.7% (atezolizumab + mFOLFOX6) vs 62.1% (mFOLFOX6 alone); manageable.
ATOMIC provides the First definitive evidence to incorporate immunotherapy into adjuvant care for dMMR colon cancer. Sets a new biomarker-driven standard.
VERIFY Trial: Rusfertide Reduces Phlebotomy Burden in Polycythemia Vera
VERIFY is Phase 3, double-blind, placebo-controlled trial in 293 Polycythemia vera patients dependent on phlebotomy. Patients randomized 1:1 to weekly subcutaneous rusfertide or placebo for 32 weeks. Stratified by use of concurrent cytoreductive therapy (CRT).
Primary Endpoint: Proportion of patients phlebotomy-free between Weeks 20–32.
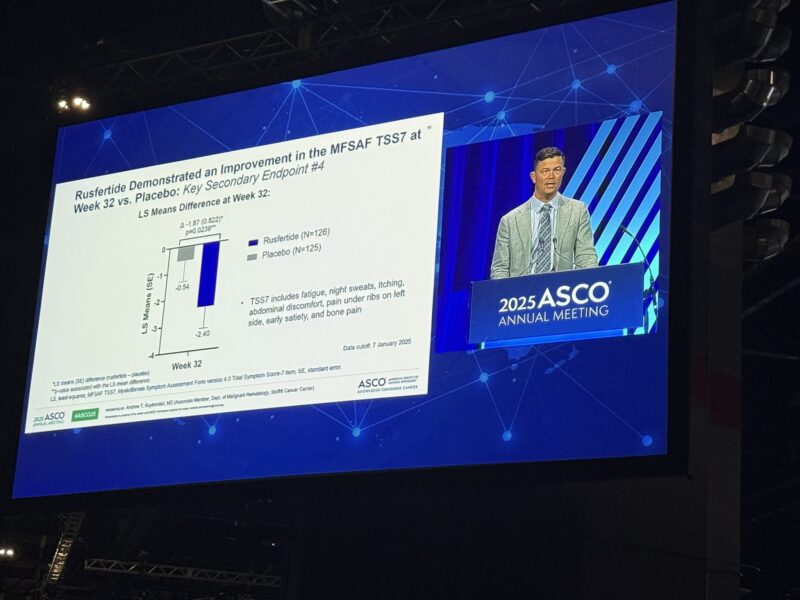
Key Findings
- Phlebotomy-free: 76.9% with rusfertide vs 32.9% with placebo (p<0.0001)
- Fewer total phlebotomies (mean: 0.5 vs 1.8)
- Hct <45%: 62.6% vs 14.4%
- Improved fatigue and symptom scores (PROMIS Fatigue, MFSAF v4.0)
Most common AEs: injection site reactions, anemia. No unexpected toxicity.
Rusfertide is the first investigational drug to show dual benefit in hematologic control and symptom improvement in PV.
NIVOPOSTOP Trial: Nivolumab Enhances DFS in High-Risk Resected LA-SCCHN
Design: Phase 3, open-label trial (N=666) in patients with resected, high-risk LA-SCCHN (oral cavity, oropharynx, hypopharynx, larynx). Patients received standard CRT (cisplatin + 66 Gy RT) with or without concurrent and maintenance nivolumab.
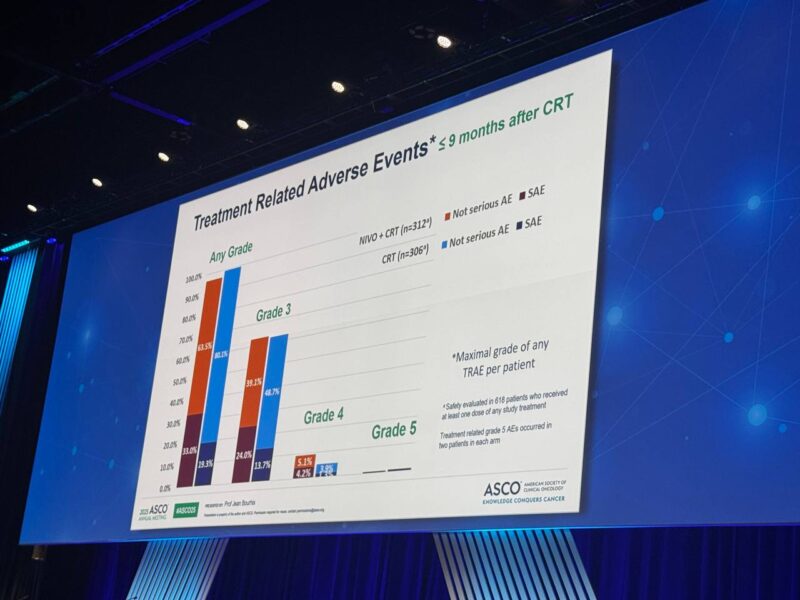
Primary Endpoint: Disease-free survival (DFS)
Key Findings from NIVOPOSTOP Tria at ASCO 2025 Plenary
- 3-year DFS: 63.1% (nivolumab + CRT) vs 52.5% (CRT alone), HR 0.76; p=0.034
- Benefit seen across PD-L1 subgroups
- Grade 4 AEs modestly increased (13.1% vs 5.6%); no new safety signals.
NIVOPOSTOP is the First advance beyond CRT in 20+ years for resected high-risk LA-SCCHN. Nivolumab + CRT is a new adjuvant standard.
SERENA-6 Trial: Camizestrant Guided by ctDNA Outperforms AI in ESR1-Mutant Breast Cancer
SERENA-6 is Phase 3, biomarker-guided trial in HR+/HER2– advanced breast cancer. Over 3,200 patients on AI + CDK4/6i underwent serial ctDNA monitoring for ESR1 mutations. Upon detection (without radiologic progression), 315 patients were randomized 1:1 to switch to camizestrant + CDK4/6i or continue AI + CDK4/6i.
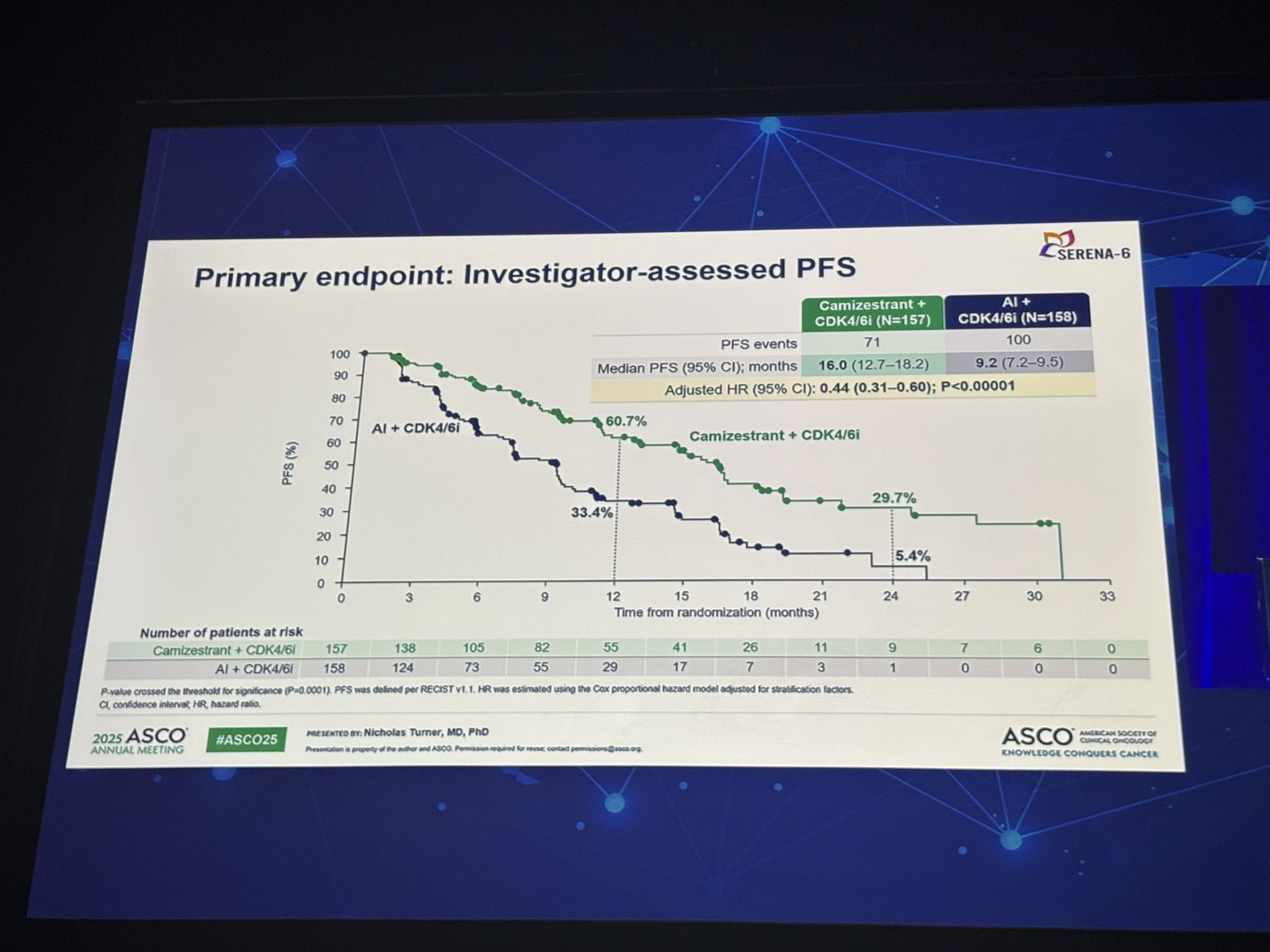
Primary Endpoint is Progression-free survival (PFS).
Key Findings From SERENA-6 presented at ASCO 2025 Plenary
- Median PFS: 16.0 months (camizestrant) vs 9.2 months (AI), HR 0.44; p<0.00001
- 12-month PFS: 60.7% vs 33.4%; 24-month: 29.7% vs 5.4%
Overall, it was well-tolerated; low discontinuation (1.3% vs 1.9%) and consistent AE profile. SERENA-6 is the First trial validating ctDNA-guided treatment to delay endocrine resistance. Camizestrant offers a proactive, precision-based switch.
ASCO 2025 Plenary Session underscores a new era in personalized and immunotherapy-driven cancer care. Trials like ATOMIC, VERIFY, MATTERHORN, SERENA-6, and NIVOPOSTOP pave the way for biomarker-based decisions, real-time surveillance, and enhanced survival outcomes across multiple cancer types. These data are expected to rapidly influence clinical guidelines and reshape standard-of-care paradigms worldwide.

Find More OncoDaily Highlights from ASCO 2025


