For more than two decades, the standard adjuvant treatment for patients with resected locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN) at high risk of relapse has been cisplatin-based chemoradiotherapy (CRT) following surgery. However, many patients still experience disease recurrence, highlighting the need for improved adjuvant strategies.
Study Design and Methods
The international, randomized, open-label NIVOPOSTOP Phase 3 trial (NCT03576417) enrolled patients under age 75 with resected LA-SCCHN of the oral cavity, oropharynx, hypopharynx, or larynx and high-risk pathological features (such as nodal extracapsular extension, positive margins, ≥4 nodal involvements, or extensive peri-neural invasion). Patients were randomized to receive either standard CRT alone (66 Gy radiation plus three cycles of cisplatin) or nivolumab added to CRT, with continued nivolumab maintenance after CRT. The primary endpoint was disease-free survival (DFS), with key secondary endpoints including overall survival (OS) and safety.
Results
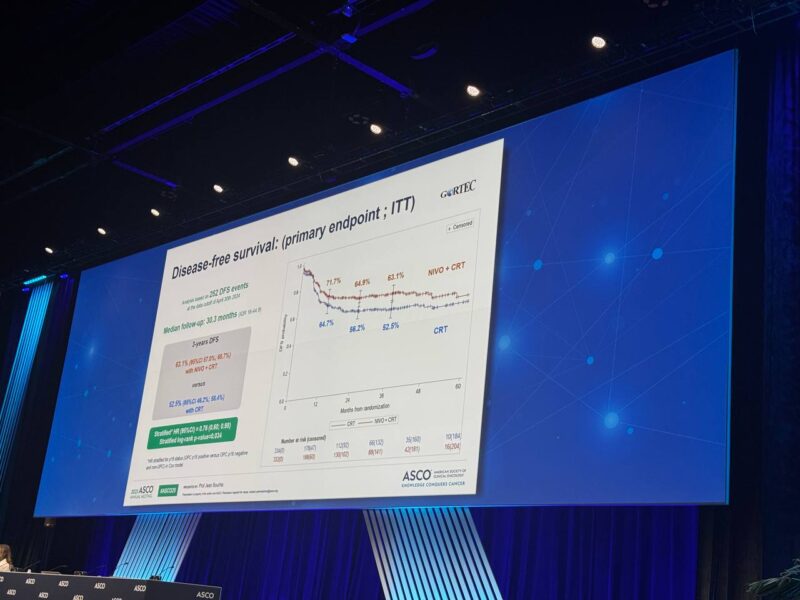
At a median follow-up of 30.3 months, analysis included 666 patients in the intent-to-treat population. Baseline characteristics were balanced between the two arms. The trial demonstrated a statistically significant improvement in DFS with adjuvant nivolumab plus CRT compared to CRT alone (hazard ratio 0.76, 95% CI: 0.60–0.98; p = 0.034). Three-year DFS rates were 63.1% for the nivolumab plus CRT group versus 52.5% for CRT alone. The benefit was consistent across PD-L1 expression subgroups. OS analysis is ongoing and will be reported after further follow-up.
Safety and Tolerability
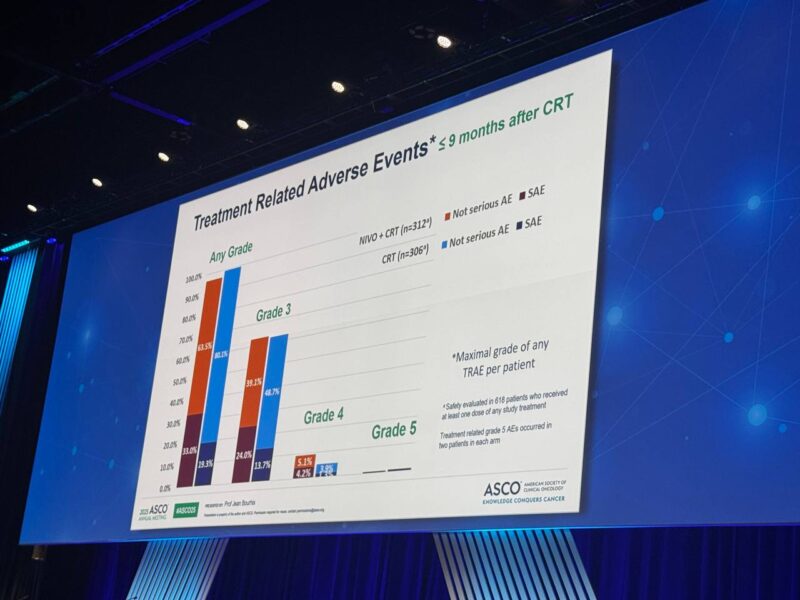
CRT compliance was similar in both study arms. Grade 4 adverse events were somewhat more frequent in the nivolumab plus CRT group (13.1%) versus CRT alone (5.6%) up to 100 days post-CRT; after this period, the rates were 1.2% and 0%, respectively. Treatment-related deaths were rare and similar (0.6% for nivolumab plus CRT; 0.7% for CRT alone). No unexpected safety signals were reported, and overall tolerability was considered acceptable.
Clinical Impact
NIVOPOSTOP is the first trial in over 20 years to demonstrate improved outcomes over standard adjuvant CRT in high-risk, resected LA-SCCHN. These results support the use of adjuvant nivolumab in combination with CRT as a new standard of care for patients with high-risk features after surgery.
Conclusion
The NIVOPOSTOP Phase 3 trial marks a significant advance in the adjuvant treatment of patients with resected, high-risk locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN). Adding nivolumab to standard cisplatin-based chemoradiotherapy resulted in a statistically and clinically meaningful improvement in disease-free survival compared to CRT alone, without unexpected safety concerns. These results establish adjuvant nivolumab plus CRT as a new standard of care for patients with high-risk features after surgery, offering new hope for improved long-term outcomes in this challenging population. Further follow-up will clarify the impact on overall survival.

Key Takeaways from the NIVOPOSTOP Phase 3 Trial
The NIVOPOSTOP Phase 3 trial demonstrated that adding adjuvant nivolumab to standard cisplatin-based chemoradiotherapy (CRT) after surgery significantly improves disease-free survival (DFS) for patients with resected, high-risk locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN). Three-year DFS rates were substantially higher in the nivolumab plus CRT group (63.1%) compared to CRT alone (52.5%), translating to a 24% reduction in the risk of recurrence or death. This benefit was consistent across all patient subgroups, including those with varying PD-L1 expression levels. The combination regimen maintained an acceptable safety and tolerability profile, with manageable rates of grade 4 adverse events and rare treatment-related deaths in both study arms. The NIVOPOSTOP trial is the first in over two decades to demonstrate a therapy that outperforms standard adjuvant CRT in this high-risk population, setting a new standard of care for patients with resected LA-SCCHN at elevated risk of recurrence.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


