The TRIPLET-HCC trial (NCT05665348), a French multicenter, prospective phase 2/3 study, investigated whether adding the CTLA-4 inhibitor ipilimumab (IPI) to the established combination of atezolizumab (ATEZO) and bevacizumab (BEV) could improve outcomes in patients with unresectable hepatocellular carcinoma (uHCC). The results of the phase 2 portion, presented at the ESMO Congress 2025, provided critical insight into the balance between efficacy and safety for this triplet immunotherapy regimen in the first-line setting.
Background
The treatment landscape for advanced or unresectable hepatocellular carcinoma has undergone remarkable evolution in recent years, driven largely by the success of immune-checkpoint inhibitors and VEGF-targeted agents. Landmark phase 3 trials—IMbrave150 (atezolizumab + bevacizumab), HIMALAYA (durvalumab + tremelimumab), and CheckMate-9DW (nivolumab + ipilimumab)—have established dual immune- or immune-anti-angiogenic blockade as the cornerstone of first-line therapy. Nevertheless, outcomes remain suboptimal for many patients, and the question of whether intensifying immune activation could extend survival persists.
The TRIPLET-HCC trial was conceived to explore this hypothesis. By introducing low-dose ipilimumab (1 mg/kg) to the atezolizumab–bevacizumab backbone, investigators aimed to test whether enhanced dual-checkpoint inhibition could increase tumor response rates and promote durable remissions without compromising safety.
Methods
In this open-label, randomized phase 2 study, 226 patients with previously untreated uHCC were enrolled between March 2023 and September 2024 across 36 centers in France. Participants were randomized 1:1 into two treatment arms:
- Arm A: ATEZO 1200 mg + BEV 15 mg/kg + IPI 1 mg/kg every 3 weeks (four doses)
- Arm B: ATEZO 1200 mg + BEV 15 mg/kg every 3 weeks
Treatment continued for up to two years or until progression or unacceptable toxicity.
The primary endpoint of phase 2 was objective response rate (ORR ≥ 35%) in Arm A within the first 24 weeks, assessed per RECIST 1.1 by investigators. Achieving this target would allow advancement to the phase 3 stage, where overall survival (OS) between arms would serve as the primary endpoint. Secondary endpoints included progression-free survival (PFS), duration of response (DoR), and safety per NCI CTC v4.0.

Results
The study did not meet its primary endpoint. In the modified intention-to-treat analysis, ORR reached 30.1% (80% CI 24.4–36.3) in Arm A, below the predefined 35% threshold required for success, compared with 27.4% (80% CI 22.0–33.5) in Arm B.
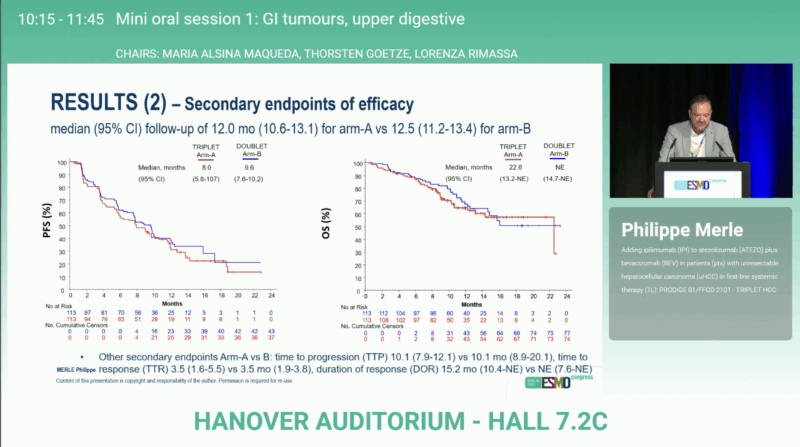
At a median follow-up of approximately 12 months, survival outcomes appeared comparable between arms:
- Median OS: 22.6 months (95% CI 13.2–NE) vs NE (95% CI 14.7–NE)
- Median PFS: 8.0 months vs 9.6 months
- Duration of response: 15.2 months vs NE (7.6-NE)
Safety analyses revealed higher toxicity with the triplet regimen. Grade 3–4 treatment-related adverse events (TRAEs) occurred in 44.2% vs 38.9% of patients, while treatment discontinuation due to toxicity was more frequent with the triplet (14.2 % vs 7.1 %). Notably, six grade 5 TRAEs were reported in Arm A compared with none in Arm B. The spectrum of adverse events was consistent with known immune- and VEGF-related toxicities, without new safety signals.
Overall, efficacy outcomes were similar, but tolerability favored the atezolizumab–bevacizumab doublet, reinforcing the robustness of this combination as standard of care.
You can read the full abstract here.
Conclusions
The phase 2 TRIPLET-HCC trial failed to demonstrate an efficacy advantage from adding low-dose ipilimumab to atezolizumab–bevacizumab in first-line unresectable HCC. Although the triplet approach was biologically rational, it did not translate into higher response rates or improved early survival outcomes and was associated with greater toxicity.
These findings reaffirm atezolizumab + bevacizumab as the benchmark first-line regimen for uHCC and suggest that further dose optimization or patient selection would be necessary before re-evaluating CTLA-4–based triplet strategies. Investigators emphasized the importance of ongoing follow-up to assess long-term survival and the potential for a subset of durable responders, outcomes that may help clarify the true contribution of ipilimumab to this combination.

The results underscore a central lesson in modern oncology: more immune activation does not always mean better outcomes. Rational combination design, informed by biomarkers and mechanistic insight, remains crucial to advancing progress in hepatocellular carcinoma.

Read more about AtezoTRIBE and AVETRIC Results at ESMO 2025: AI-Powered Histopathology Predicts Immunotherapy Benefit in pMMR mCRC on OncoDaily.