Post-treatment surveillance after curative surgery for colorectal cancer (CRC) is a cornerstone of patient management, yet current guidelines rely more on expert consensus than robust evidence. Intensive follow-up programs—including regular CT scans and carcinoembryonic antigen (CEA) testing—are believed to improve early detection of recurrence, but their real impact on overall survival (OS) remains debated. The PRODIGE-13 trial aimed to provide clarity by rigorously evaluating the value of intensive imaging and CEA monitoring in stage II–III CRC patients.
Does Intensive Imaging and CEA Monitoring Improve Outcomes?
The PRODIGE-13 trial was launched to answer a long-standing clinical question: can more intensive post-surgical surveillance improve survival in patients with stage II–III colorectal cancer (CRC)? Current follow-up recommendations rely largely on expert consensus rather than robust data, and involve regular imaging and carcinoembryonic antigen testing.
What Are the Current Guideline Recommendations?
According to the NCCN Guidelines Version 4.2025 for Colon Cancer still favor structured surveillance with both CT imaging and CEA monitoring for patients with resected stage II–III disease. Specifically, CEA testing is recommended every 3–6 months for the first 2 years, then every 6 months until 5 years post-surgery. This approach is intended to catch asymptomatic recurrences early, enabling potentially curative interventions such as metastasectomy.
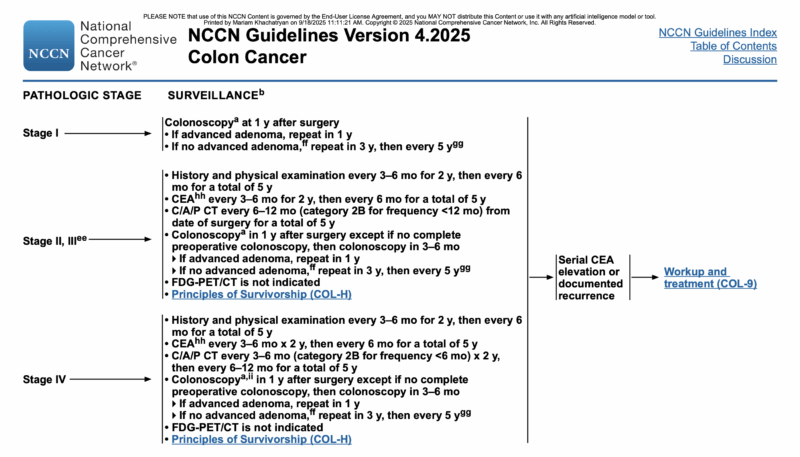
For imaging, the guidelines advise chest, abdominal, and pelvic CT scans every 6–12 months for up to 5 years, with the aim of detecting metastatic relapse before it becomes unresectable. In patients who undergo resection of stage IV disease, the intensity of imaging increases, with CT scans recommended every 3–6 months for the first two years, followed by every 6–12 months thereafter.
What is PRODIGE-13 Trial?
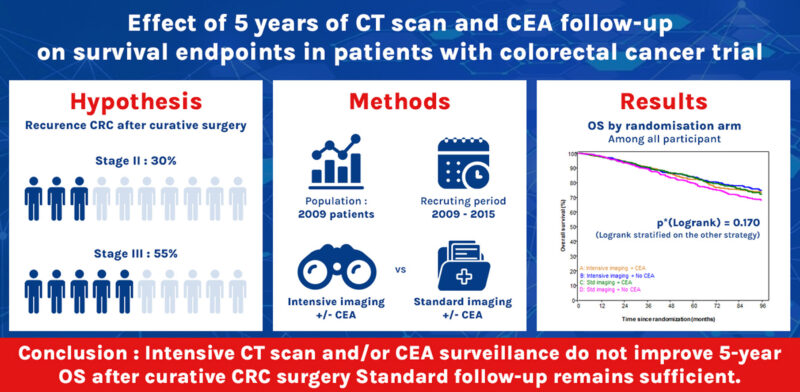
This prospective, multicenter phase III trial enrolled 2,009 patients from France and Belgium who had undergone curative surgery with no residual disease on baseline imaging. Participants were randomized into four groups in a factorial design:
- Group A: Intensive imaging (alternating abdominal ultrasound/CT every 3 months) + CEA testing
- Group B: Intensive imaging only
- Group C: Standard imaging (US every 3 months + chest X-ray every 6 months) + CEA testing
- Group D: Standard imaging only
Endpoints
The primary endpoint was 5-year overall survival (OS). Secondary endpoints included relapse-free survival (RFS) and recurrence patterns. Median follow-up was 7.8 years.
Key Results of PRODIGE-13 trial
Among the 2,009 randomized patients, 16% had rectal cancer and 44% had left-sided colon cancer. Most patients (75.9%) were younger than 75 years at inclusion. During follow-up, cancer recurred in 22.3% of patients, with 10.5% experiencing local recurrence, 72.9% metastatic recurrence, and 16.6% both.
Five-year OS rates
- 82.1% (95% CI: 78.5–85.2) in Group A
- 84.1% (95% CI: 80.5–87.0) in Group B
- 83.6% (95% CI: 80.1–86.6) in Group C
- 79.5% (95% CI: 75.7–82.8) in Group D
The difference across groups was not statistically significant (log-rank p = 0.170). Median OS was not reached in any arm.
Five-year RFS
- 73.8% in the CT-scan surveillance group vs. 69.3% in the no-CT group (HR 0.89; 95% CI: 0.76–1.03; p = 0.108)
- 71.3% in the CEA surveillance group vs. 71.8% in the no-CEA group (HR 1.00; 95% CI: 0.86–1.16; p = 0.959)
These findings, published in Annals of Oncology (ESMO) on September 17, 2025, suggest that neither CT-scan based intensive surveillance nor routine CEA monitoring improves survival outcomes. The results may have an important impact on clinical practice by supporting a shift toward more cost-effective, streamlined follow-up strategies without compromising patient outcomes.
Takeaway
This phase III study underscores that intensive CT and CEA surveillance may not be necessary for all patients following curative surgery for stage II–III colorectal cancer. Future research should focus on risk-adapted follow-up, guided by molecular and clinical prognostic factors.

You can also read about Top Colorectal Cancer Trials to Watch at ESMO 2025 on OncoDaily.