Colorectal cancer (CRC) is a malignancy originating in the colon or rectum, components of the large intestine. It stands as one of the most prevalent cancers globally and is a leading cause of cancer-related mortality, particularly in developed nations. According to GLOBOCAN 2020 estimates, over 1.9 million new CRC cases and more than 930,000 deaths occurred worldwide in that year, accounting for approximately 10% of all cancer incidences and 9.4% of cancer deaths.
The incidence of CRC exhibits significant geographical variation, with the highest rates observed in regions such as Australia, New Zealand, and parts of Europe, and the lowest rates in regions like Africa and South-Central Asia . Notably, while the overall incidence of CRC has been declining in older adults, largely due to effective screening programs, there has been a concerning rise in early-onset CRC cases—diagnoses occurring in individuals under 50 years of age. Studies indicate that early-onset CRC incidence increased overall from 2001 to 2016, with variations observed across different racial and ethnic groups.
This article aims to provide a comprehensive overview of colorectal cancer, encompassing current scientific knowledge on its risk factors, molecular pathogenesis, clinical presentation, diagnostic strategies, staging, treatment approaches, and emerging therapies. By understanding these aspects, we can better address the challenges posed by CRC and improve outcomes for those affected.
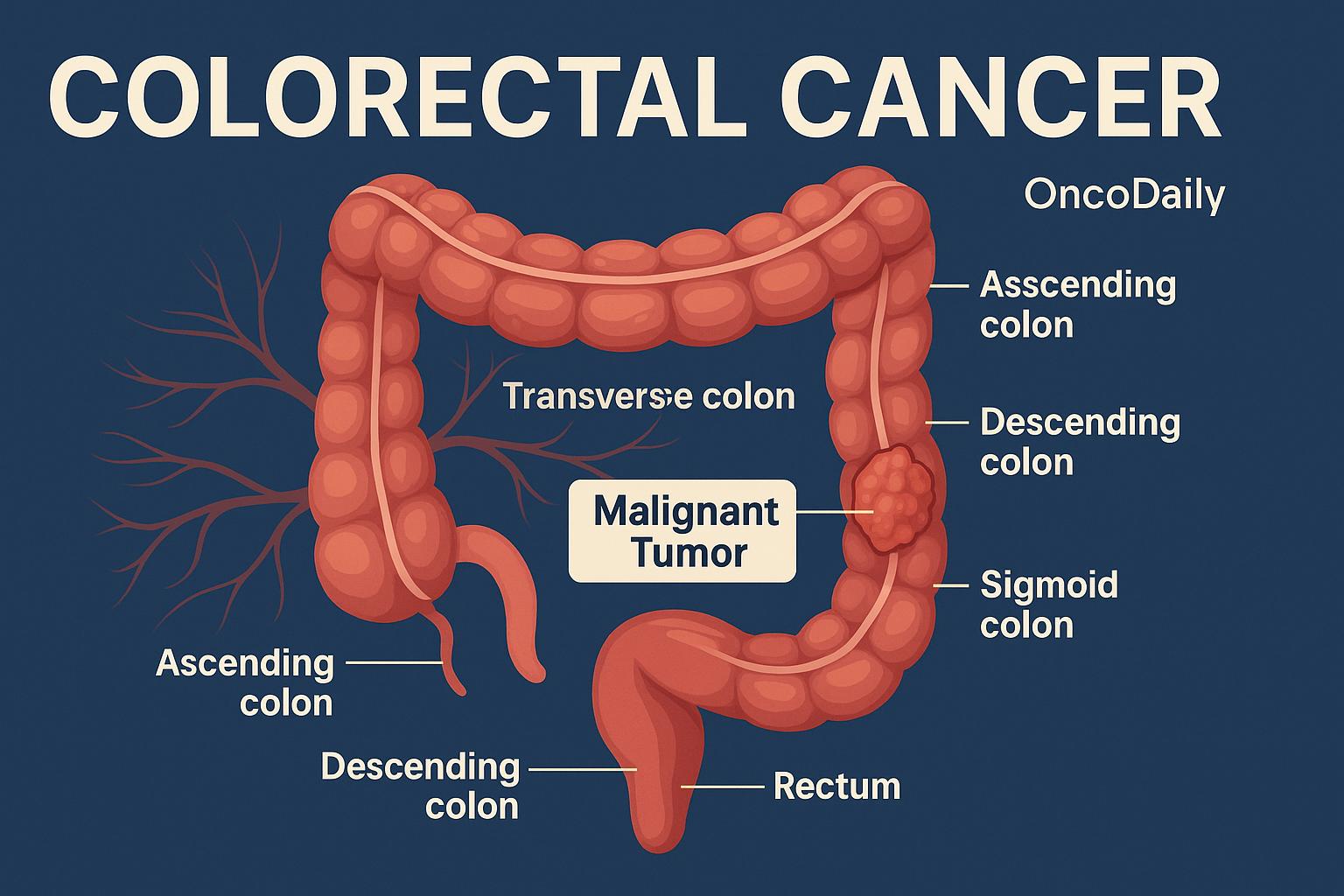
Classification of Colorectal Cancer: Anatomical, Histological, Molecular, and Staging Perspectives
Colorectal cancer (CRC) represents a biologically and clinically diverse group of tumors. To guide diagnosis, prognosis, and treatment, it is categorized through several intersecting classification systems, each reflecting different aspects of tumor behavior—ranging from anatomical location to histopathologic features, molecular signatures, and clinical stage.
From an anatomical standpoint, CRC is broadly divided based on the site of origin within the colon or rectum. Tumors arising in the right colon—including the cecum and ascending colon—tend to have distinct biological characteristics compared to those from the left colon, such as the descending or sigmoid segments. Right-sided tumors are more often associated with mucinous histology, high levels of microsatellite instability (MSI), and a poorer overall prognosis. In contrast, left-sided cancers are more likely to present with obstructive symptoms and are generally characterized by chromosomal instability. Rectal cancers, while technically part of left-sided disease, have unique considerations for staging and treatment due to their location and proximity to pelvic structures.
In terms of histological classification, the vast majority of colorectal tumors are adenocarcinomas, though important subtypes exist. Conventional adenocarcinomas, forming glandular structures, are the most prevalent. Mucinous adenocarcinomas, defined by extracellular mucin comprising over 50% of the tumor volume, tend to be diagnosed at a more advanced stage and exhibit resistance to standard chemotherapy. Rarer yet more aggressive are signet-ring cell carcinomas, characterized by intracellular mucin that displaces the nucleus, often associated with diffuse invasion and poorer clinical outcomes.
At the molecular level, colorectal cancers are further defined by distinct genetic and epigenetic alterations. One of the most significant distinctions is between microsatellite instability-high (MSI-H) and microsatellite stable (MSS)tumors. MSI-H cancers, which account for about 15% of CRCs, arise due to defects in DNA mismatch repair genes and are particularly relevant due to their responsiveness to immunotherapy. Additionally, the Consensus Molecular Subtypes (CMS) framework, established through gene expression profiling, identifies four subtypes of CRC: CMS1 (MSI-immune), CMS2 (canonical), CMS3 (metabolic), and CMS4 (mesenchymal). Each subtype reflects a distinct biology with implications for prognosis and therapeutic response.
Beyond MSI and CMS classification, several key oncogenic mutations guide treatment decisions. KRAS and NRASmutations, present in a substantial subset of patients, predict resistance to anti-EGFR monoclonal antibodies. BRAF V600E mutations, often found in MSI-H tumors, are associated with worse outcomes but have become targets for emerging combination therapies. Meanwhile, HER2 amplification and NTRK fusions, although rare, offer actionable targets in selected patients, expanding the landscape of precision medicine in CRC.
Finally, staging remains critical in determining treatment intent and prognosis. The AJCC/UICC TNM staging system (8th edition) categorizes tumors based on the extent of primary invasion (T), regional lymph node involvement (N), and presence of distant metastases (M). Early-stage tumors confined to the bowel wall may be curable with surgery alone, while more advanced stages require multidisciplinary management, including chemotherapy, radiation, and targeted therapies.
Risk Factors and Etiological Pathways in Colorectal Cancer
Colorectal cancer (CRC) is influenced by a combination of non-modifiable and modifiable risk factors, as well as underlying inflammatory conditions. Understanding these factors is crucial for effective prevention and early detection strategies.
Non-Modifiable Risk Factors
- Age is a significant determinant in CRC risk. The incidence of CRC increases markedly after the age of 50, with the majority of cases diagnosed in individuals over this age threshold. However, recent trends indicate a concerning rise in early-onset CRC among younger populations.
- Family history also plays a pivotal role. Individuals with a first-degree relative diagnosed with CRC have a higher risk compared to those without such a history. A meta-analysis encompassing 27 studies found that a family history of CRC is associated with a 2.21-fold increased risk (OR 2.21; 95% CI 1.54–3.17) .
- Inherited syndromes contribute significantly to CRC risk. Lynch syndrome, also known as hereditary non-polyposis colorectal cancer (HNPCC), is the most common hereditary CRC syndrome, accounting for approximately 2% to 4% of all CRC cases. This condition results from inherited mutations in DNA mismatch repair genes, leading to microsatellite instability . Familial adenomatous polyposis (FAP) is another hereditary condition characterized by the development of numerous polyps in the colon and rectum, with a near 100% risk of progressing to CRC if left untreated.
Modifiable Risk Factors
- Dietary habits significantly influence CRC risk. High consumption of red and processed meats has been consistently associated with increased CRC incidence. A comprehensive meta-analysis reported that each 100 g/day increase in red and processed meat intake is associated with a 12% increased risk of CRC .
- Obesity is another critical factor. Excess body weight, particularly central adiposity, has been linked to higher CRC risk. A systematic review and meta-analysis found that obesity is associated with a 1.52-fold increased risk of early-onset CRC (OR 1.52; 95% CI 1.20–1.91) .
- Lifestyle choices such as smoking and alcohol consumption further exacerbate CRC risk. Smoking has been associated with a 1.44-fold increased risk (OR 1.44; 95% CI 1.10–1.88), while alcohol consumption is linked to a 1.41-fold increased risk (OR 1.41; 95% CI 1.22–1.62) .
- Physical inactivity contributes to CRC development as well. Sedentary behavior has been associated with a 1.24-fold increased risk (OR 1.24; 95% CI 1.05–1.46) .
Inflammatory Conditions
Chronic inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn’s disease, are well-established risk factors for CRC. Individuals with long-standing ulcerative colitis or Crohn’s colitis face an approximately 2–3-fold increased risk of developing CRC. The risk escalates with the duration and extent of the disease .
Clinical Presentation of Colorectal Cancer
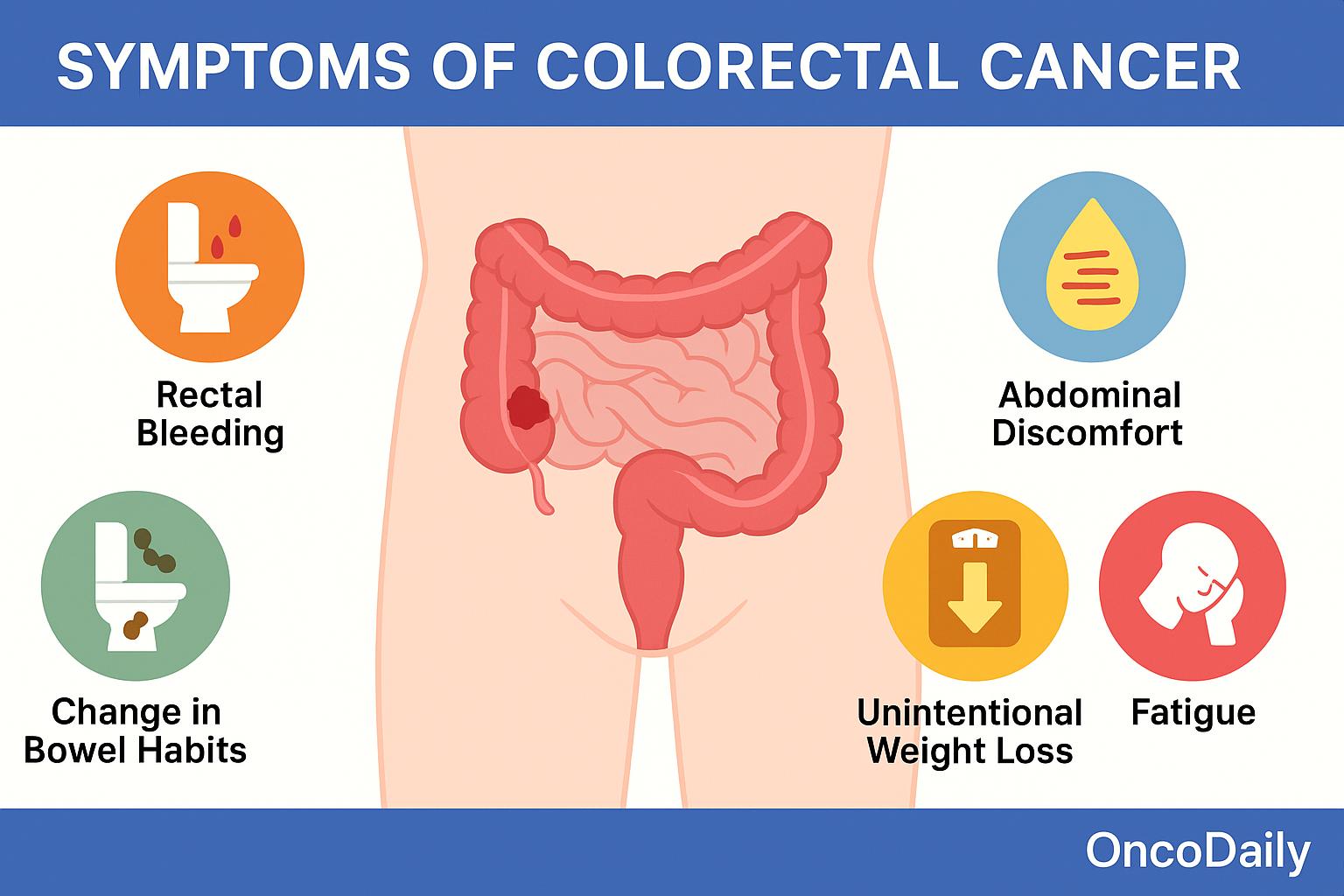
Colorectal cancer (CRC) can present with a broad range of clinical symptoms, many of which vary depending on the tumor’s location within the colon or rectum. However, in its earliest stages, CRC is often asymptomatic, making regular screening essential for early detection. Left-sided tumors, which include cancers of the descending colon, sigmoid colon, and rectum, tend to produce symptoms that reflect the narrower lumen and firmer stool consistency in this portion of the bowel. Patients often report changes in bowel habits, such as alternating constipation and diarrhea, rectal bleeding, or hematochezia (bright red blood in stool). As the tumor grows, it may cause partial or complete bowel obstruction, leading to cramping, abdominal distension, and urgent, painful attempts to defecate.
In contrast, right-sided tumors, arising from the cecum and ascending colon, usually present more insidiously. These cancers are more likely to cause occult (hidden) gastrointestinal bleeding, which may go unnoticed until it leads to iron-deficiency anemia. Patients may experience fatigue, pallor, or vague abdominal discomfort. Due to the larger diameter of the right colon and the more liquid nature of stool, right-sided tumors are less likely to cause early obstructive symptoms, often resulting in delayed diagnosis.
When CRC advances beyond the local stage, systemic symptoms may appear. Patients with advanced or metastatic disease can experience unintentional weight loss, persistent fatigue, or even a palpable abdominal mass on physical examination. Additional signs may arise depending on the sites of metastasis—for example, jaundice in liver involvement or respiratory symptoms in lung metastases.
Given that early-stage colorectal cancer often develops without noticeable symptoms, many cases are only detected through routine screening methods such as colonoscopy or fecal immunochemical testing (FIT). This reality emphasizes the critical importance of population-wide screening programs, particularly for individuals over the age of 45 or those with risk factors such as a family history or inflammatory bowel disease. Early detection through screening significantly improves prognosis and expands treatment options.
Diagnosis of Colorectal Cancer
The diagnosis of colorectal cancer (CRC) involves a comprehensive approach that combines screening techniques, imaging studies, histologic confirmation, and increasingly, molecular profiling. This integrated method enables early detection, accurate staging, and personalized treatment planning.
In asymptomatic individuals, CRC is often first detected through screening programs. Colonoscopy remains the gold standard, providing full visualization of the colon and rectum and allowing for simultaneous biopsy or removal of suspicious polyps. Other screening tools include the fecal immunochemical test (FIT)—a non-invasive assay for occult blood with good sensitivity and specificity—and stool DNA testing, which combines FIT with molecular markers associated with neoplasia. Flexible sigmoidoscopy offers a partial endoscopic option, and for patients unable to undergo standard colonoscopy, CT colonography (virtual colonoscopy) is a validated alternative.
Once a lesion is identified, histological confirmation via biopsy is required. This is usually obtained during diagnostic colonoscopy. The majority of CRCs are adenocarcinomas, but the pathology report also provides important prognostic details such as tumor grade, lymphovascular invasion, and mucinous or signet-ring features.Accurate imaging is essential for staging. A contrast-enhanced CT scan of the chest, abdomen, and pelvis is routinely used to detect distant metastases, particularly in the liver, lungs, and peritoneum. For rectal cancer, pelvic MRI is the modality of choice for local staging, offering detailed evaluation of tumor depth, mesorectal fascia involvement, and nodal status.
Molecular testing is now a critical component of CRC diagnosis and management. Both NCCN and ESMO guidelines recommend specific molecular evaluations, particularly in patients with metastatic or recurrent disease. These include:
-
KRAS and NRAS mutations, which predict resistance to anti-EGFR monoclonal antibodies (cetuximab, panitumumab).
-
BRAF V600E mutations, associated with poorer prognosis and now targetable with BRAF/MEK/EGFR inhibitors.
-
HER2 amplification, relevant in RAS/BRAF wild-type tumors and targetable with HER2-directed therapies.
-
NTRK fusions, rare but actionable targets in select cases, responsive to TRK inhibitors.
-
Circulating tumor DNA (ctDNA), emerging as a tool for minimal residual disease assessment and early relapse detection, though not yet standard for all cases.
Importantly, microsatellite instability (MSI) or mismatch repair (MMR) testing is recommended for all patients with CRC, regardless of stage. This has both prognostic and therapeutic implications. MSI-high or dMMR tumors are typically associated with better prognosis in early-stage disease and a high likelihood of response to immune checkpoint inhibitors (e.g., pembrolizumab) in advanced disease. Universal testing also plays a critical role in identifying patients with Lynch syndrome, the most common hereditary CRC syndrome.
Treatment Strategies for Colorectal Cancer: A Stage-Based Narrative Overview
The management of colorectal cancer (CRC) is highly individualized and depends on the tumor’s location, stage, and molecular characteristics, as well as the patient’s overall health and treatment goals. Modern treatment strategies follow the evidence-based recommendations outlined in the NCCN Guidelines, incorporating a combination of surgery, chemotherapy, radiation, targeted therapy, and immunotherapy across different stages of disease.
For early-stage disease (stages I–III), surgical resection remains the primary treatment and is often curative. In stage I and low-risk stage II colon cancer, surgery alone is typically sufficient. However, in high-risk stage II cases—identified by factors such as T4 lesions, lymphovascular invasion, or poorly differentiated histology—and in stage III, adjuvant chemotherapy is recommended to reduce the risk of recurrence. Two chemotherapy regimens are commonly used: FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) and CAPOX (capecitabine and oxaliplatin). The choice between them depends on patient preferences, toxicity profiles, and logistical considerations such as oral versus infusion-based therapy. Both regimens have demonstrated efficacy in improving disease-free and overall survival in node-positive colon cancer.
In stage IV (metastatic) disease, treatment becomes more complex and typically centers on systemic therapy, with a goal of prolonging survival, relieving symptoms, and, in select cases, rendering metastases resectable. Initial chemotherapy options include FOLFOX, FOLFIRI (which substitutes irinotecan for oxaliplatin), and FOLFOXIRI (a triplet combination used in younger patients with good performance status).
These regimens are often combined with targeted therapies. For example, the anti-angiogenic agent bevacizumab is used to inhibit tumor vascularization, while anti-EGFR monoclonal antibodies like cetuximab or panitumumab are effective in patients with left-sided, RAS and BRAF wild-type tumors. The importance of molecular stratification was highlighted in the FIRE-3 trial, which demonstrated that adding cetuximab to FOLFIRI significantly improved overall survival in patients with RAS wild-type, left-sided metastatic colorectal cancer compared to bevacizumab-based therapy.
Patients with MSI-H (microsatellite instability-high) or dMMR (deficient mismatch repair) tumors represent a biologically distinct subset that responds exceptionally well to immune checkpoint inhibitors. The KEYNOTE-177 trial showed that pembrolizumab monotherapy resulted in a median progression-free survival of 16.5 months versus 8.2 months for standard chemotherapy in treatment-naïve MSI-H/dMMR metastatic CRC, leading to its adoption as a first-line option in these patients.
For patients with potentially resectable metastatic disease, particularly isolated liver or lung metastases, multidisciplinary evaluation is essential. Some patients may benefit from systemic therapy aimed at downsizing metastases to enable surgical resection. The CAIRO5 trial is one example exploring conversion strategies, demonstrating that carefully selected patients may achieve long-term survival through combined modality treatment.
The approach to rectal cancer is notably distinct due to the anatomical and functional challenges associated with tumors in the pelvic region. For locally advanced rectal cancer (T3/T4 or node-positive), the current standard involves neoadjuvant therapy to reduce tumor size and improve the likelihood of complete surgical resection.
Traditionally, this consisted of chemoradiation, but newer protocols such as Total Neoadjuvant Therapy (TNT), which integrates both chemotherapy and chemoradiation before surgery, are gaining favor due to improved pathological complete response rates and lower rates of distant relapse. Following neoadjuvant therapy, patients typically undergo total mesorectal excision (TME), a specialized surgical technique that minimizes local recurrence. For selected early-stage rectal tumors, local excision may be appropriate, particularly if the tumor is T1 and lacks high-risk features.
In all cases, adjuvant therapy decisions after surgery depend on pathological findings, tumor regression after neoadjuvant treatment, and patient-specific risk factors.
Emerging Therapies and Personalized Medicine in Colorectal Cancer
Liquid biopsies, particularly the analysis of circulating tumor DNA (ctDNA), are emerging as valuable tools in colorectal cancer for detecting minimal residual disease (MRD) and monitoring treatment response. Studies have shown that fluctuations in ctDNA levels correlate with recurrence risk and may inform decisions about adjuvant therapy, especially in stage II and III disease. According to recent reports in ESMO Daily Reporter, ctDNA-guided management is entering prospective clinical validation, potentially redefining postoperative surveillance.
Beyond recurrence prediction, ctDNA testing offers a non-invasive means of identifying actionable mutations in real-time, enabling clinicians to tailor targeted therapies for patients with metastatic CRC. These tools are being incorporated into both research and clinical practice to personalize care across the disease continuum.
HER2 amplification, found in approximately 2–3% of metastatic CRC cases—especially in RAS/BRAF wild-type tumors—has emerged as a therapeutic target. The combination of tucatinib and trastuzumab has received FDA approval for patients with previously treated HER2-positive CRC, offering a new treatment avenue backed by clinical benefit and durable responses (cancer.gov). Similarly, KRAS G12C mutations, present in about 3–4% of CRC cases, are now targetable with novel small-molecule inhibitors. The FDA recently granted accelerated approval to adagrasib in combination with cetuximab for patients with previously treated KRAS G12C-mutant metastatic CRC, based on encouraging objective response and disease control rates (ASCO Daily News).
While immunotherapy has transformed the treatment landscape for MSI-high CRC, the majority of patients (≈95%) with MSS tumors have shown limited benefit from current immune checkpoint inhibitors. However, novel dual checkpoint blockade strategies are beginning to shift this paradigm.
One promising development is the combination of botensilimab, a next-generation Fc-enhanced CTLA-4 inhibitor, with balstilimab, an anti–PD-1 antibody. This immunotherapy pairing is designed to amplify T-cell activation while reducing immune suppression, aiming to sensitize MSS tumors to immune attack. In a phase 1/2 study (Nature Medicine, 2024), the botensilimab/balstilimab regimen showed an objective response rate (ORR) of 17% and disease control rate (DCR) of 61% in heavily pretreated MSS CRC patients. Importantly, patients without liver metastases experienced higher response rates, suggesting that metastatic site biology may influence immunotherapy efficacy.
In the UNICORN trial, which explored this combination in the neoadjuvant setting, the pathological complete response rate reached 29%, with major pathological responses observed in 36% of cases—an encouraging signal for potential future use in organ preservation strategies.
Innovations in artificial intelligence (AI) and multi-omics integration are driving a shift toward predictive, biology-based treatment models. By analyzing combinations of genomic, transcriptomic, epigenomic, and proteomic data, researchers are developing models that stratify CRC patients by recurrence risk, treatment sensitivity, and immune landscape (PMC). These tools may help clinicians not only choose optimal therapies but also predict which patients may respond to emerging agents like KRAS inhibitors or immunotherapy combinations.
Beyond checkpoint blockade, other experimental immunotherapies are being explored for CRC. Cancer vaccines, designed to stimulate tumor-specific immune responses, are undergoing early-phase testing in both adjuvant and metastatic settings. Meanwhile, CAR-T cell therapy, which has shown success in hematologic malignancies, is being adapted for solid tumors. Targets such as Claudin18.2 and CEA are under active investigation for CRC, though challenges such as tumor microenvironment resistance remain.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is colorectal cancer (CRC)?
Colorectal cancer is a malignancy that originates in the colon or rectum, often developing from precancerous polyps and influenced by genetic, environmental, and lifestyle factors.
What are the main risk factors for CRC?
Major risk factors include age over 50, family history, inherited syndromes like Lynch syndrome, obesity, smoking, alcohol use, diets high in red/processed meat, and inflammatory bowel disease.
What are the symptoms of colorectal cancer?
Symptoms may include changes in bowel habits, blood in the stool, abdominal pain, fatigue, anemia, and unexplained weight loss. Right-sided and left-sided CRC may present differently.
How is CRC diagnosed?
Diagnosis involves colonoscopy with biopsy, imaging (CT, MRI, PET), and molecular testing for mutations (KRAS, NRAS, BRAF, MSI). Screening tools like FIT are used for early detection.
What does MSI-high mean in colorectal cancer?
MSI-high indicates high microsatellite instability due to mismatch repair deficiency. These tumors often respond well to immunotherapy and may indicate Lynch syndrome.
What are the treatment options for CRC?
Treatment varies by stage and may include surgery, chemotherapy (e.g., FOLFOX, FOLFIRI), radiation (especially for rectal cancer), targeted therapies (e.g., anti-EGFR, anti-VEGF), and immunotherapy.
What is ctDNA and how is it used in CRC?
Circulating tumor DNA (ctDNA) is used for non-invasive detection of minimal residual disease and relapse monitoring. It’s also being explored for treatment personalization.
Are there targeted therapies for CRC?
Yes. HER2 amplification, KRAS G12C mutations, BRAF V600E, and NTRK fusions are targetable with specific therapies. Molecular testing is essential for guiding treatment.
What is total neoadjuvant therapy (TNT) in rectal cancer?
TNT involves delivering both chemotherapy and radiation before surgery to improve tumor response, allow organ preservation, and reduce recurrence.
What advancements are emerging in CRC treatment?
New developments include AI-guided decision-making, ctDNA monitoring, next-gen immunotherapies (e.g., botensilimab + balstilimab), cancer vaccines, and CAR-T for solid tumors.