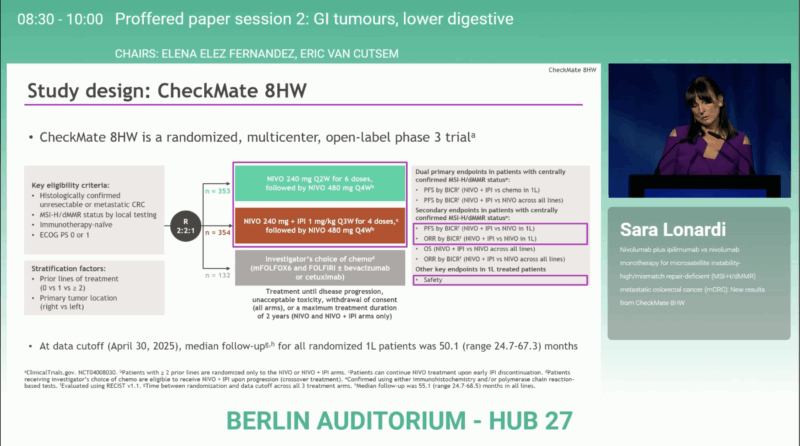
At the ESMO Congress 2025 in Berlin, Dr. Sara Lonardi (Padua, Italy) presented new and definitive results from the phase III CheckMate 8HW trial (LBA29, NCT04008030), comparing nivolumab (NIVO) plus ipilimumab (IPI)versus nivolumab monotherapy in patients with microsatellite-instability–high/mismatch-repair–deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC).
This long-term analysis confirmed that dual PD-1/CTLA-4 blockade delivers durable efficacy gains across lines of therapy with manageable safety, reinforcing its position as the standard of care for this biologically distinct population.
Background
MSI-H/dMMR colorectal cancer represents roughly 5% of all metastatic CRC cases and is characterized by a hyper-mutated phenotype that confers high sensitivity to immune checkpoint inhibition. Early trials established nivolumab alone as an effective therapy; however, adding ipilimumab—a CTLA-4 inhibitor—was hypothesized to induce broader T-cell activation and deepen tumor responses.
CheckMate 8HW is the largest randomized study in MSI-H/dMMR mCRC, designed to evaluate whether dual checkpoint blockade offers incremental benefit beyond PD-1 monotherapy and to benchmark its efficacy against chemotherapy. The study previously met both of its dual primary endpoints of superior progression-free survival (PFS)versus chemotherapy in the first-line setting and versus nivolumab across all lines. The newly presented analysis, with data cutoff April 30 2025, provides the final PFS results and updated overall survival (OS) and objective response rate (ORR) outcomes.
Study Design
Patients with unresectable or metastatic MSI-H/dMMR CRC were randomized (2:2:1) to receive:
- Nivolumab + Ipilimumab (NIVO + IPI),
- Nivolumab (NIVO) alone, or
- Chemotherapy ± targeted therapy (CT).
Centrally confirmed MSI-H/dMMR status was required for the primary efficacy analyses. The dual primary endpoints—progression-free survival (PFS) in the first-line setting and across all therapy lines—had previously been met. The newly presented data include the final PFS analysis (cutoff: April 30 2025) and updated overall survival (OS) and objective response rate (ORR) findings.
Results
With a median follow-up of 55.1 months (range 24.7–68.5), nivolumab + ipilimumab continued to demonstrate meaningful and durable benefit over nivolumab alone in centrally confirmed MSI-H/dMMR mCRC.
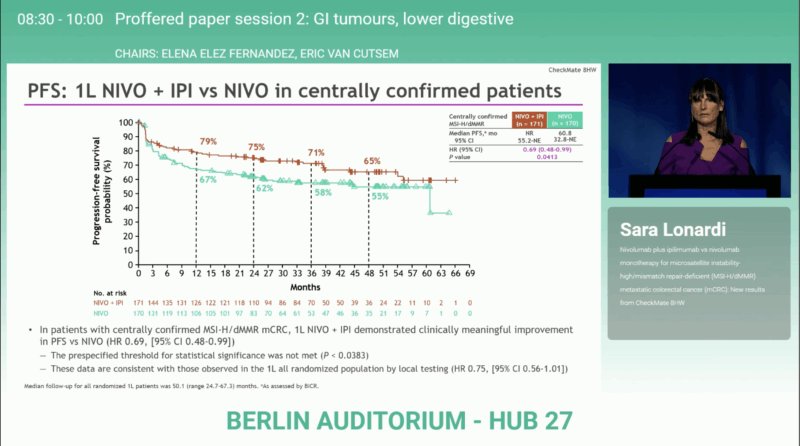
First-line population (n = 171 vs 170):
- Median PFS: Not reached (55.2–NE) with NIVO + IPI vs 60.8 months (32.8–NE) with NIVO
→ HR 0.69 (95% CI 0.48–0.99); P = 0.0413, Although the difference was clinically meaningful, the prespecified statistical boundary for significance (P < 0.0383) was not crossed. - Objective response rate: 73% (95% CI 65–79) vs 61% (95% CI 53–69). Among first-line patients, complete responses occurred in 35% vs 31%, partial responses in 37% vs 31%, stable disease in 12% vs 19%, and progressive disease in 11% vs 16% for NIVO + IPI and NIVO, respectively.
Median time to response was 2.8 months (range 1.2–38.6) with NIVO + IPI and 2.7 months (1.2–29.5) with NIVO.
Median duration of response was not reached in either arm (95% CI not estimable for NIVO + IPI vs 59.4–NE for NIVO).
Across all therapy lines (n = 296 vs 286):
- Median OS: Not reached in either arm; HR 0.61 (95% CI 0.45–0.83). OS probabilities at 12, 24, 36, and 48 months were 90%, 85%, 81%, and 78% with NIVO + IPI, compared with 83%, 72%, 69%, and 65% with NIVO. At the time of analysis, only 69% of expected events (168 of 243 deaths) had occurred, indicating that OS data remain immature.
- Median PFS: Not reached vs 44.3 months (22.1–NE); HR 0.62 (95% CI 0.48–0.80)
- ORR: 71% vs 58%
These data confirm a 31% reduction in the risk of progression or death and a 39% reduction in the risk of death overall with dual checkpoint blockade compared with PD-1 monotherapy.
Safety
The safety profile of the combination remained predictable and manageable. Any-grade treatment-related adverse events (TRAEs) occurred in 82% of patients receiving NIVO + IPI and 78% with NIVO monotherapy. Grade 3–4 TRAEs were reported in 24% vs 17%, and serious TRAEs in 19% vs 10%, respectively. Treatment discontinuations due to TRAEs occurred in 16% vs 8%, and treatment-related deaths were reported in 2 (1%) vs 1 (<1%) patients. The most frequent TRAEs (≥10%) with NIVO + IPI included pruritus (24%), diarrhea (21%), hypothyroidism (16%), asthenia (14%), fatigue (13%), rash (12%), adrenal insufficiency (10%), and ALT increase (10%). No new safety signals were observed with longer follow-up.
Discussion
These results confirm that combining PD-1 and CTLA-4 blockade deepens tumor regression and prolongs disease control beyond what PD-1 inhibition alone can achieve. The 8HW data also address lingering questions regarding the durability of dual immunotherapy: after more than four years of follow-up, both PFS and OS curves remain separated and plateauing, signaling sustained benefit in a high proportion of responders.
The incremental toxicity associated with ipilimumab is balanced by significant long-term survival advantage, making nivolumab + ipilimumab the preferred regimen for fit patients with MSI-H/dMMR mCRC, while nivolumab monotherapy remains an option for those with contraindications to combination immunotherapy.
Both PFS and OS curves remained separated and plateaued beyond 48 months, with approximately 65% of patients on NIVO + IPI progression-free at four years compared with 55% on NIVO alone.
You can read the full abstract here.
Conclusion
The CheckMate 8HW final PFS analysis reinforces the long-term efficacy and tolerability of nivolumab plus ipilimumab over nivolumab alone in MSI-H/dMMR metastatic colorectal cancer. With a 31% lower risk of progression, 39% lower risk of death, and higher response rates sustained over nearly five years, the regimen establishes dual immune checkpoint blockade as the benchmark standard of care in this population.
You can read about PEGASUS Trial at ESMO 2025: Liquid Biopsy-Guided Adjuvant Therapy in Resected Colon Cancer on OncoDaily.