Biliary tract cancer (BTC) is an aggressive malignancy where timely molecular profiling is critical to guide therapy. Despite the availability of targeted treatments for alterations such as FGFR2 fusions, IDH1 mutations, and HER2 amplifications, roughly one-quarter of patients never undergo genotyping. Circulating cfDNA testing offers a non-invasive alternative, but its true clinical and prognostic value has not been fully established.
This article explores how circulating cell-free DNA is reshaping BTC management. It reviews the latest data on cfDNA’s accuracy compared to tissue sequencing, its prognostic value, and its role in tracking resistance. It also looks at how advances in targeted therapy, immunotherapy, and ongoing clinical trials are changing patient outcomes in 2025.
What Is cfDNA?
Circulating cell-free DNA refers to small fragments of DNA released into the bloodstream from dying cells, including tumor cells. When sequenced using next-generation sequencing (NGS), cfDNA — commonly called a “liquid biopsy” — can reveal the molecular profile of a cancer without requiring tissue samples. This approach is particularly attractive for BTC, where tumors can be difficult to biopsy and tissue is often scarce.
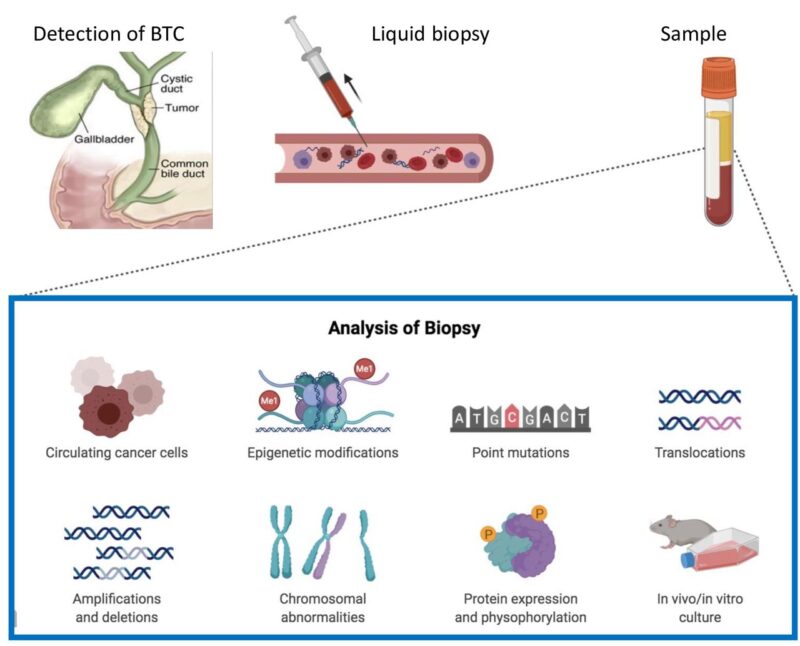
source: CANCER GENOMICS & PROTEOMICS
Understanding Biliary Tract Cancer
Biliary tract cancer (BTC) includes cholangiocarcinoma (bile duct cancer) and gallbladder cancer. These are rare but aggressive malignancies often diagnosed late because early symptoms are subtle or absent. As a result, many patients are not candidates for curative surgery at the time of diagnosis. Diagnosis relies on a combination of blood tests, imaging (ultrasound, CT, MRI/MRCP), and sometimes endoscopic or percutaneous procedures to obtain tissue samples. Tumor markers like CA 19-9 and CEA can support suspicion but are not definitive. Once confirmed, staging determines treatment strategy.
Prognosis remains poor, with five-year survival rates under 10% for advanced disease. Even after surgery, recurrence is common. The treatment landscape now combines surgery (where possible) with systemic chemotherapy, targeted therapy for actionable mutations (FGFR2 – Futibatinib, Pemigatinib, IDH1-Ivosidenib, HER2 – Zanidatamab IHC3+, BRAF), immunotherapy, radiation, and locoregional techniques like Y-90 radioembolization.
This growing focus on molecular profiling has made precision medicine an integral part of BTC care. Identifying tumor mutations can open the door to targeted treatments — and this is where cell-free DNA, or “liquid biopsy,” is emerging as a key tool to overcome tissue limitations and monitor tumor evolution over time.
Study Overview
The investigation analyzed 297 cfDNA samples from 170 patients with BTC and compared them with matched tumor tissue using targeted next-generation sequencing (NGS). The study assessed the frequency of actionable genomic alterations, concordance between plasma and tissue sequencing, prognostic value of cfDNA detection in resected and metastatic disease, and molecular evolution at progression on targeted therapy.
Results
The most common genomic alterations identified in cfDNA were TP53 (29%), FGFR2 (16%), ARID1A (13%), CDKN2A (11%), and KRAS (11%). Importantly, one in four patients had OncoKB level 1/2 actionable alterations, and over one-third of potentially actionable variants were found exclusively in plasma, highlighting the added value of cfDNA testing. The concordance between plasma and tissue sequencing was remarkably high, reaching 96% for IDH1, 98% for BRAF, 92% for KRAS, 99% for ERBB2 amplifications, and 96% for FGFR2 fusions.
From a prognostic standpoint, the presence of detectable tumor-derived cfDNA after resection did not predict recurrence. However, in treatment-naïve metastatic BTC, a high variant allele fraction was associated with significantly worse progression-free and overall survival. Notably, RAS alterations that were absent at baseline emerged in 24% of patients at progression on BRAF-, FGFR-, or HER2-targeted therapies, identifying a convergent mechanism of acquired resistance.
These results were published in JCO Precision Oncology on September 16, 2025.
Conclusion
This study demonstrates that cfDNA-based NGS is a robust, complementary tool to tissue sequencing for the identification of actionable genomic alterations in BTC. It provides crucial insights into tumor biology, detects resistance mechanisms at progression, and may guide therapy selection when tissue is unavailable or insufficient. These findings support broader integration of cfDNA testing into routine clinical practice for patients with biliary tract cancer.