Zanidatamab (Ziihera) is an innovative bispecific antibody designed to target HER2-expressing cancers with high precision. As a next-generation HER2-directed therapy, it represents a promising option for patients whose tumors have shown resistance to traditional HER2-targeted treatments. Backed by emerging clinical trial data and regulatory progress, Zanidatamab is shaping the future of HER2-positive cancer care across multiple tumor types. This article will explore Zanidatamab’s current and potential uses in cancer, its side effects, recommended dosage, and what patients can expect during treatment.
Which company produced Zanidatamab?
Zanidatamab (Ziihera®) was developed by Zymeworks Inc., a Canadian biotech company known for its bispecific antibody platform, Azymetric™, which enables dual binding to HER2 and enhances anti-tumor activity. To expand access, Zymeworks partnered with key industry players. In 2022, Jazz Pharmaceuticals acquired global development and commercialization rights (excluding parts of Asia) and now leads its U.S. regulatory and clinical efforts. In Asia-Pacific regions, including China, rights were licensed to BeiGene (now BeOne Medicines), which achieved conditional approval in China in 2025.
Additionally, Jazz Pharmaceuticals has partnered with MD Anderson Cancer Center to explore zanidatamab across HER2-expressing tumors and with ALX Oncology to study combination strategies enhancing immune response. These collaborations support the global development of zanidatamab across a range of HER2-positive cancers.

Learn more about Cholangiocarcinoma: Latest Risk Factors, Symptoms, Diagnosis, and 2025 Advanced Treatment Options for Bile Duct Cancer on OncoDaily.
How does Zanidatamab Work?
Zanidatamab is a bispecific HER2-targeted monoclonal antibody designed to bind two distinct extracellular domains (ECD2 and ECD4) of the HER2 receptor. This dual binding promotes enhanced receptor clustering, internalization, and degradation, leading to downstream inhibition of HER2 signaling pathways implicated in tumor cell growth and survival.
Unlike conventional HER2-directed antibodies such as trastuzumab (which binds ECD4) or pertuzumab (which targets ECD2), zanidatamab’s simultaneous engagement of both domains facilitates a unique mechanism of action that results in:
- Increased antibody-dependent cellular cytotoxicity (ADCC)
- Enhanced receptor internalization and downregulation
- Disruption of HER2 dimerization and downstream signaling
- Increased tumor cell phagocytosis via immune effector cell recruitment
This bispecific design allows zanidatamab to overcome resistance mechanisms seen in tumors with heterogeneous or low HER2 expression, broadening its potential efficacy across multiple HER2-expressing tumor types, including gastroesophageal adenocarcinoma, breast cancer, biliary tract cancer, and others. Clinical studies have shown that zanidatamab exhibits favorable pharmacokinetics, sustained target engagement, and robust anti-tumor activity both as monotherapy and in combination regimens. Its safety profile is consistent with other HER2-targeted therapies, with manageable adverse events such as diarrhea and infusion-related reactions.
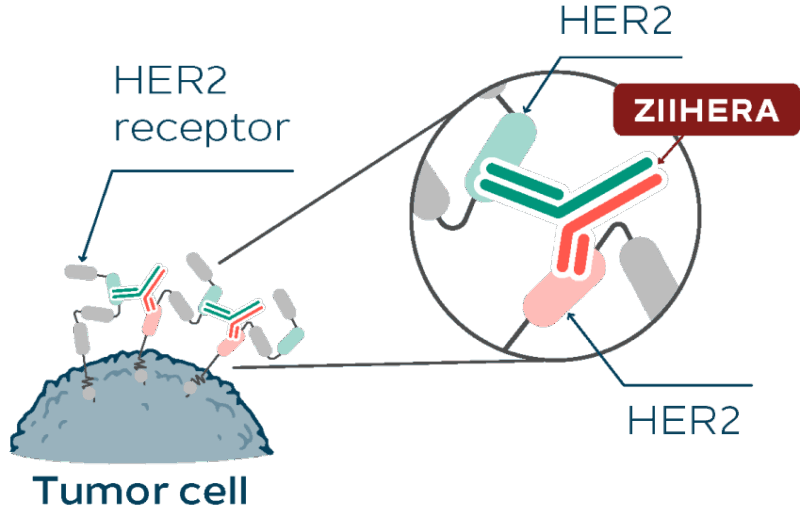
Source: Ziihera® Official Website
What Cancers Is Zanidatamab Approved to Treat?
In November 2024, the FDA granted accelerated approval to Ziihera (zanidatamab-hrii) for adults with previously treated, inoperable or metastatic HER2-positive biliary tract cancer. Developed by Jazz Pharmaceuticals, Ziihera is given as an intravenous injection and targets cancers with strong HER2 protein expression (IHC 3+).
What research is behind the approval?
The FDA granted accelerated approval to Ziihera (zanidatamab-hrii) for previously treated, unresectable or metastatic HER2-positive biliary tract cancer, based on results from the HERIZON-BTC-01 trial published in The Lancet Oncology in 2023. In this study, 87 patients whose cancer had progressed despite earlier treatment were enrolled. Most had HER2-positive tumors (IHC 2+ or 3+), and all received zanidatamab, a bispecific antibody that targets two different sites on the HER2 receptor. Patients were given 20 mg/kg intravenously every two weeks.
The results were promising. Among patients with HER2-positive tumors (cohort 1), the confirmed objective response rate (ORR)—meaning their tumors shrank significantly—was 41.3%. Responses were seen across different subtypes of biliary tract cancer. The median duration of response was 12.9 months, and the disease control rate (including those whose cancer stabilized) was 68.8%. Median progression-free survival (PFS) was 5.5 months, and overall survival (OS) reached a median of 12.0 months. The treatment was generally well tolerated. Grade 3 treatment-related side effects occurred in 18% of patients. The most common were diarrhea (5%) and decreased ejection fraction (3%). No patients experienced grade 4 side effects or treatment-related deaths.
00242-5/asset/7fc32553-d07e-4937-9c6a-5355746f0881/main.assets/gr2a.jpg)
Its safety profile, published by the FDA in 2024, showed that Ziihera was generally well tolerated in patients with HER2-positive biliary tract cancer. In the HERIZON-BTC-01 trial, the treatment achieved a 52% objective response rate, with a median duration of response of 14.9 months. The most common side effects were diarrhea, infusion-related reactions, abdominal pain, and fatigue.
Zanidatamab side effects and its management
Like many cancer treatments, zanidatamab can cause side effects. These occur because the drug not only targets cancer cells but may also affect healthy cells in the body. Understanding the possible side effects and how to manage them can help patients stay as comfortable as possible during treatment.
Common side effects
The most frequently observed adverse effects include hematologic and biochemical abnormalities, infusion-related reactions, and gastrointestinal symptoms. Decreased hemoglobin, reflecting anemia, occurs in a majority of patients, alongside elevated lactate dehydrogenase and lowered albumin levels. Liver enzymes such as AST and ALT are commonly increased, indicating hepatic stress. Gastrointestinal issues such as diarrhea, abdominal pain, nausea, vomiting, and decreased appetite are reported with notable frequency. Infusion-related reactions manifest as symptoms like fever, chills, flushing, or shortness of breath during or shortly after drug administration. Fatigue and rash are also relatively common and may impact patients’ quality of life.
Less Common Side Effects
Less common but clinically significant side effects include left ventricular dysfunction, which requires careful monitoring due to its potential impact on cardiac function. Pneumonitis, although rare, is a serious adverse event that necessitates permanent discontinuation of therapy when confirmed at Grade 2 or higher. Electrolyte imbalances such as hyponatremia and hypokalemia may occur, requiring close laboratory monitoring. Zanidatamab has not been extensively studied in patients with moderate to severe hepatic or renal impairment, so caution is warranted in these populations.
Management of Side Effects
Premedication with acetaminophen, antihistamines such as diphenhydramine, and corticosteroids like hydrocortisone is recommended 30 to 60 minutes before each infusion to reduce the risk and severity of infusion-related reactions. For mild infusion reactions, slowing the infusion rate and gradual re-escalation is advised. Moderate reactions require pausing the infusion and symptom treatment, with careful resumption at a reduced rate. Severe reactions necessitate stopping the infusion and often lead to permanent discontinuation if recurrent.
Diarrhea management depends on severity. Mild to moderate diarrhea is treated supportively without dose modification, while severe diarrhea may require temporary treatment interruption and dose reduction upon improvement. Persistent or prolonged severe diarrhea despite optimized care warrants permanent discontinuation.
Cardiac monitoring is essential before and during treatment. Therapy should be withheld if significant decreases in left ventricular ejection fraction occur, with treatment resuming only upon recovery. Symptomatic heart failure or persistent cardiac dysfunction mandates permanent discontinuation. Other adverse reactions of moderate to severe grade require treatment interruption until improvement, with dose reductions considered upon resumption. Persistent Grade 4 toxicities typically necessitate stopping zanidatamab permanently.

What is the Recommended Dosage of Zanidatamab?
Zanidatamab is given as a 300 mg powder for IV infusion, dosed at 20 mg/kg every two weeks for previously treated HER2-positive biliary tract cancer. Premedication with acetaminophen, antihistamines, and corticosteroids is recommended to reduce infusion reactions. If side effects occur, the dose can be lowered to 15 mg/kg; if still not tolerated, treatment should stop. Heart function is closely monitored, and treatment is paused or stopped based on changes in ejection fraction or heart failure symptoms.
How is Zanidatamab administered?
Zanidatamab is given as an intravenous (IV) infusion. Before each dose, patients are premedicated with acetaminophen, an antihistamine, and a corticosteroid to help prevent infusion-related reactions. The drug is mixed with sterile water and then diluted in a saline or dextrose solution. It should be clear and free of particles before use. The infusion is filtered and must not be given as a quick injection or mixed with other IV drugs in the same line.
Infusion times depend on how well the patient tolerated previous doses:
- First 1–2 infusions: about 2 hours
- Infusions 3–4: about 90 minutes
- Later infusions: about 1 hour
Unopened vials are stored in the fridge. Once prepared, the solution must be used within a limited time, depending on whether it’s kept at room temperature or refrigerated.
What to Avoid During Zanidatamab Treatment?
While receiving Zanidatamab, it’s important to follow certain precautions to ensure the treatment is safe and effective. Patients should avoid taking any new medications, including over-the-counter drugs or herbal supplements, without first speaking to their doctor, as some may interfere with how the treatment works or increase the risk of side effects.
Alcohol should be limited or avoided, especially if it worsens any side effects like fatigue, nausea, or liver irritation. It’s also best to avoid vaccines—especially live vaccines—during treatment, unless approved by the healthcare provider, as the immune system may be weakened.
Patients should not drive or operate heavy machinery during or shortly after the infusion if they feel dizzy, lightheaded, or unwell. Finally, it’s essential to avoid missing doses or delaying infusions without medical guidance, as this could reduce the drug’s effectiveness. Regular communication with the care team, staying hydrated, and resting well between infusions can help support overall well-being throughout the treatment course.
Zanidatamab effectiveness over time
A four-year follow-up of a phase 2 trial evaluating zanidatamab plus chemotherapy in HER2-positive advanced or metastatic gastroesophageal adenocarcinoma (mGEA) showed promising long-term results. Published in the Journal of Clinical Oncology in 2025, the study reported that the combination treatment delivered a median overall survival (OS) of 36.5 months, with durable responses and a manageable safety profile.
The trial included 46 patients receiving zanidatamab with various chemotherapy backbones (mFOLFOX6, CAPOX, or FP). Most had centrally confirmed HER2-positive tumors. The objective response rate was 76%, and median progression-free survival reached 12.5 months. Notably, plasma ctDNA levels dropped significantly early in treatment, reflecting potential for molecular response monitoring. After prophylactic measures were introduced, the incidence of severe diarrhea was reduced from 52% to 24%. No treatment-related deaths were reported.
These findings reinforce zanidatamab’s role as a potent first-line therapy in HER2-positive mGEA, with survival benefits exceeding three years in many patients.

Learn more about Stomach Cancer: Symptoms and Causes, Types, Diagnosis and Treatment on OncoDaily.
Ongoing trials with Zanidatamab
A Phase 2 trial (DiscovHER PAN-206, NCT06695845) is currently evaluating zanidatamab in patients with previously treated solid tumors that overexpress HER2 (IHC 3+). Sponsored by Jazz Pharmaceuticals, the open-label, multicenter study includes various cancer types, such as breast, gastric, colorectal, ovarian, lung, and pancreatic cancers. The goal is to assess the efficacy and safety of zanidatamab in this diverse, HER2-expressing patient population.
FAQ
What type of drug is Zanidatamab?
Zanidatamab is a bispecific HER2-targeted antibody. Unlike traditional HER2 therapies that bind only one site, it binds two domains (ECD2 and ECD4) on the HER2 receptor, leading to stronger anti-tumor activity.
Which cancers can Zanidatamab currently treat?
As of 2025, Ziihera is FDA-approved for HER2-positive biliary tract cancer and conditionally approved in China for HER2-positive cancers.
How does Zanidatamab differ from trastuzumab (Herceptin)?
Trastuzumab binds only one HER2 domain (ECD4), while Zanidatamab binds two distinct HER2 domains simultaneously.
What are the most common side effects of Zanidatamab?
The most frequent side effects include diarrhea, infusion-related reactions, abdominal pain, nausea, and fatigue.
How is Zanidatamab given to patients?
Zanidatamab is administered as an intravenous infusion every two weeks.
What should patients avoid while receiving Zanidatamab?
Patients should avoid alcohol, live vaccines, and new medications or supplements without medical advice.


