How Chemotherapy Affects Cancer and Normal Cells: Mechanisms, Side Effects and Long-Term Risks
Chemotherapy remains a cornerstone of systemic cancer treatment, with its primary mechanism rooted in the targeting of rapidly dividing cells. Developed during the mid-20th century, early chemotherapeutic agents were designed to exploit the high mitotic activity of malignant cells—a defining feature that distinguished them from most normal somatic cells. This foundational principle led to the development of cytotoxic drugs capable of interfering with DNA synthesis, mitosis, or other critical processes required for cell division.
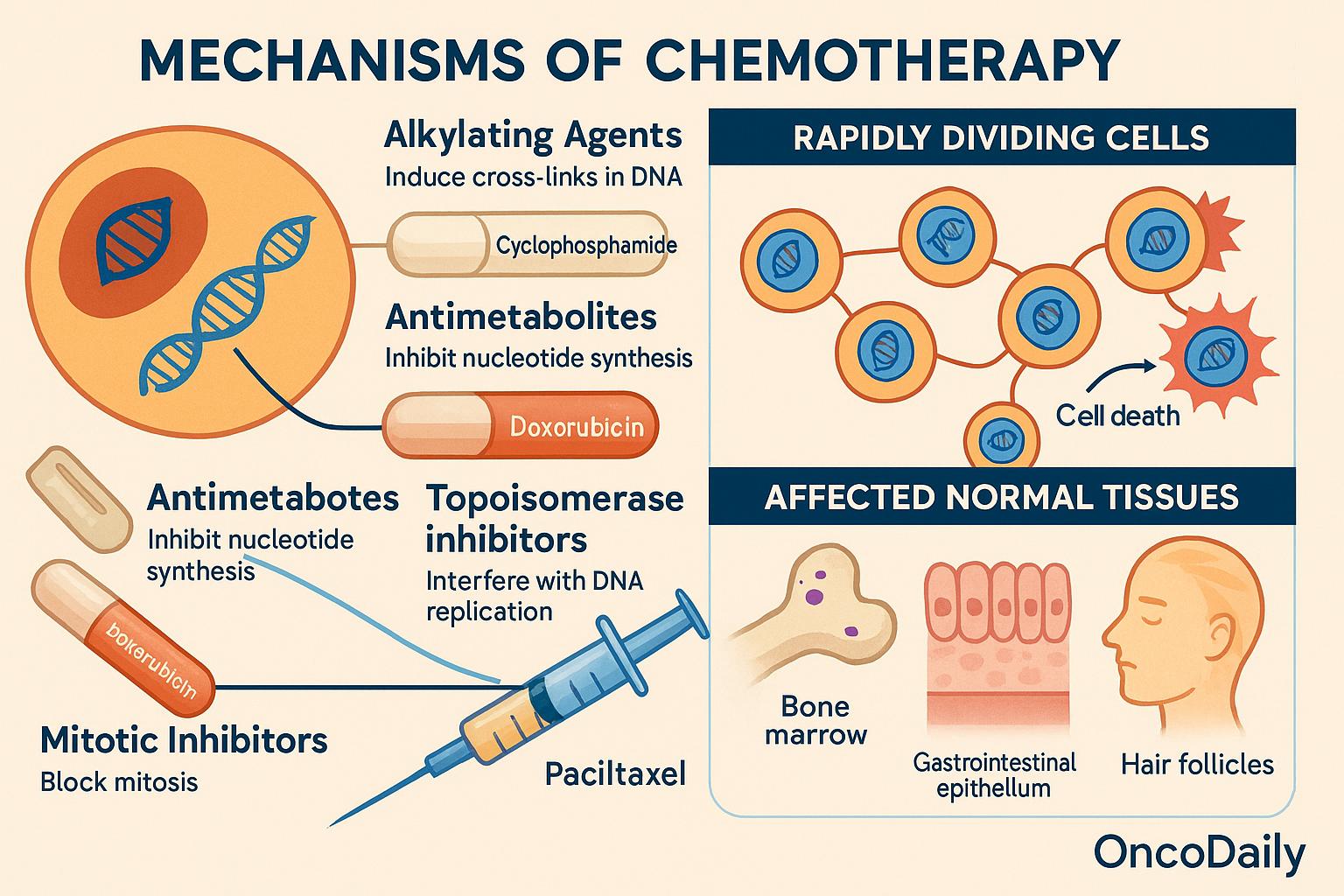
However, despite its intent to selectively eradicate cancer cells, chemotherapy lacks precise specificity. Many healthy tissues—particularly those with high cellular turnover, such as hematopoietic stem cells in the bone marrow, epithelial cells of the gastrointestinal tract, and hair follicle cells—also undergo frequent division and are thus equally susceptible to chemotherapy-induced damage. This off-target toxicity underlies the well-recognized spectrum of adverse effects associated with cytotoxic agents, ranging from myelosuppression to mucositis and alopecia.
This article explores in depth how chemotherapy exerts its effects on both cancerous and noncancerous tissues, illuminating the biological principles behind its benefits and its limitations.
Mechanisms of Action of Chemotherapeutic Agents
Chemotherapeutic agents exert their cytotoxic effects primarily by interfering with the fundamental processes of cell proliferation. These drugs are designed to disrupt DNA synthesis, mitotic progression, or nucleotide metabolism—cellular pathways that are particularly active in rapidly dividing cells. Among the principal classes, alkylating agents (such as cyclophosphamide) induce cross-links in DNA strands, impairing replication and transcription. Antimetabolites (like 5-fluorouracil and methotrexate) mimic normal substrates of nucleotide synthesis, thereby inhibiting the formation of purines or pyrimidines essential for DNA and RNA synthesis. Topoisomerase inhibitors (e.g., doxorubicin, irinotecan) prevent the unwinding and resealing of DNA strands during replication, leading to double-strand breaks and genomic instability. Finally, mitotic inhibitors (such as paclitaxel and vincristine) disrupt microtubule dynamics, arresting cells in metaphase and triggering apoptosis.
These agents do not possess inherent selectivity for malignant cells. Instead, they preferentially damage cells with high mitotic rates—a hallmark of many cancers—but a trait also shared by several normal tissues. Consequently, tissues such as the bone marrow, gastrointestinal epithelium, and hair follicles, which are characterized by constant cellular turnover, are particularly vulnerable to off-target cytotoxicity. This fundamental lack of absolute specificity underlies both the therapeutic efficacy and the dose-limiting toxicities of chemotherapy, underscoring the need for improved delivery strategies and selective targeting in modern oncologic practice.
Differential Proliferation and the Limits of Selectivity
One of the foundational principles underlying chemotherapy is differential proliferation—the concept that cytotoxic agents preferentially affect cells with high mitotic activity. Cancer cells, by their very nature, often proliferate in an unregulated and accelerated manner, making them particularly vulnerable to agents that disrupt DNA replication or mitosis. This rapid division, however, is not unique to malignant cells. Several normal tissues—most notably hematopoietic stem cells, gastrointestinal mucosal epithelium, and hair follicles—also possess high turnover rates essential for tissue renewal and homeostasis.
This overlap in proliferative behavior between malignant and normal cells contributes to the narrow therapeutic windowof traditional chemotherapeutic agents. The therapeutic index—a ratio that compares the drug dose that achieves tumoricidal effects to the dose that causes unacceptable toxicity in normal tissues—is often limited in cytotoxic chemotherapy. Unlike targeted therapies, where selectivity arises from molecular recognition (e.g., receptor overexpression or mutation-specific binding), classical chemotherapeutics rely on relative, rather than absolute, susceptibility to cell cycle disruption.
To improve selectivity and reduce off-target toxicity, contemporary oncology is exploring multiple strategies. Pharmacokinetic optimization, such as adjusting infusion duration or dosing schedules, can help modulate drug exposure in healthy tissues. Prodrug formulations are being developed that remain inactive until metabolized in the tumor microenvironment, thereby localizing cytotoxicity. Additionally, targeted delivery systems, including liposomal encapsulation and nanoparticle carriers, are being employed to concentrate drug activity within the tumor while sparing normal tissues.
Cytotoxic Effects of Chemotherapy on Cancer Cells
Chemotherapy exerts its anticancer activity by disrupting essential cellular processes that are required for proliferation and survival. The most direct consequence of many cytotoxic agents is DNA damage, whether through base alkylation, cross-link formation, or topoisomerase inhibition. Such damage compromises the integrity of the genome, triggering cell cycle checkpoints and activating intrinsic surveillance pathways. When the damage is beyond repair, cells undergo apoptosis, mitotic catastrophe, or in some cases, necrosis. A key mediator of this response is the tumor suppressor p53, which orchestrates DNA damage sensing, cell cycle arrest, and apoptotic signaling. In cancers with intact p53 function, chemotherapy-induced genotoxic stress often culminates in programmed cell death.
Another mechanism is mitotic arrest, particularly targeted by agents such as taxanes and vinca alkaloids, which interfere with microtubule dynamics. Prolonged arrest in metaphase leads to mitotic catastrophe—a form of cell death characterized by chromosomal missegregation and nuclear fragmentation. In other scenarios, particularly in hypoxic or metabolically stressed tumors, cells may succumb to necrosis, releasing pro-inflammatory signals that influence the tumor microenvironment.
However, not all cancer cells respond uniformly to chemotherapy. Tumor heterogeneity—both inter- and intratumoral—plays a critical role in shaping treatment outcomes. The mutation profile, including alterations in TP53, BCL2, or mismatch repair genes, significantly influences susceptibility to apoptosis. Similarly, the tumor microenvironment can confer resistance through hypoxia, acidosis, or stromal interactions that shield tumor cells from cytotoxic effects.
Moreover, many cancer cells acquire adaptive resistance mechanisms. These include the upregulation of anti-apoptotic proteins such as BCL-2 and survivin, which block cell death pathways even in the face of genotoxic stress. Others enhance their DNA repair capacity, as seen with increased expression of BRCA1/2, PARP, or mismatch repair enzymes. Some tumors overexpress drug efflux pumps, notably P-glycoprotein (MDR1), which actively transport chemotherapy agents out of the cell, reducing intracellular drug concentrations below therapeutic levels.
Impact of Chemotherapy on Normal Tissues: Systemic Toxicities and Dose Limitations
While chemotherapy aims to eradicate malignant cells, its lack of absolute specificity often results in significant collateral damage to healthy tissues—particularly those with high proliferative capacity or susceptibility to cellular stress. These effects span multiple organ systems and are central to the morbidity of cancer treatment. In many cases, they constitute the dose-limiting toxicities that constrain therapeutic intensity and frequency, ultimately influencing both treatment success and patient quality of life.
Hematologic toxicity is one of the most common and clinically significant adverse effects. Cytotoxic agents frequently target the bone marrow, where hematopoietic stem cells are in continuous proliferation. This results in neutropenia, which increases infection risk; anemia, contributing to fatigue and reduced oxygen delivery; and thrombocytopenia, heightening the potential for bleeding. These complications often necessitate treatment delays, dose reductions, or the use of hematopoietic growth factors such as G-CSF or erythropoietin.
The gastrointestinal system is also highly vulnerable due to the rapid turnover of epithelial cells lining the mucosa. Damage to these cells manifests as mucositis, leading to painful ulcerations and increased infection risk; diarrhea, which can be severe and dehydrating; and nausea and vomiting, among the most distressing side effects. The latter is mediated through stimulation of the chemoreceptor trigger zone and gastrointestinal tract receptors and is now mitigated to some extent with modern antiemetic regimens.
Dermatologic toxicity, particularly alopecia, arises from the disruption of rapidly growing hair follicle cells. Although not life-threatening, hair loss can have a profound psychological impact on patients, affecting self-image and social functioning.
The reproductive system is sensitive to chemotherapy, especially alkylating agents that damage the germinal epithelium in ovaries and testes. This results in gonadotoxicity, manifesting as infertility, premature ovarian failure, or hypogonadism. These effects are often irreversible and carry significant implications for younger patients, necessitating discussions around fertility preservation prior to treatment initiation.
Neurologic toxicity, most notably peripheral neuropathy, occurs with agents such as taxanes, platinums (e.g., cisplatin, oxaliplatin), and vinca alkaloids. Patients may experience paresthesia, numbness, and motor deficits, which can be dose-cumulative and occasionally permanent. These symptoms not only impair function but may also require dose reduction or discontinuation of otherwise effective therapies.
In addition, certain chemotherapeutic drugs have organ-specific toxicities. Cardiotoxicity, classically associated with anthracyclines like doxorubicin, can lead to irreversible heart failure through oxidative stress and mitochondrial injury. Nephrotoxicity, particularly with cisplatin, results from tubular injury and impaired renal clearance. Pulmonary toxicity, seen with bleomycin and busulfan, may cause pneumonitis or fibrosis, often presenting insidiously but potentially fatal.
These toxicities are not merely side effects; they are critical determinants of chemotherapy administration. The concept of the dose-limiting toxicity—the maximum dose that can be given without causing unacceptable harm—guides dosing schedules, necessitates supportive care interventions, and often determines whether a patient can continue on a given regimen. Balancing the need for tumor control with the risk of normal tissue injury is a persistent challenge in oncologic treatment planning.
DNA Damage, Repair, and the Double-Edged Nature of Chemotherapy
Many chemotherapeutic agents function by directly damaging DNA, exploiting the dependency of proliferating cells on intact genomic replication. Alkylating agents, platinum compounds, and topoisomerase inhibitors introduce various forms of DNA lesions, including strand breaks, inter- and intra-strand crosslinks, and base modifications. These disruptions activate cellular DNA damage response (DDR) pathways, which play a pivotal role in determining cell fate—either repair and survival or irreversible arrest and death.
In normal cells, the DNA repair machinery is typically intact and highly efficient. Pathways such as homologous recombination, base excision repair, nucleotide excision repair, and non-homologous end joining are activated to resolve DNA lesions and preserve genomic integrity. This robust reparative capacity often allows normal tissues to recover from sublethal damage, particularly if dosing schedules provide adequate intervals for regeneration.
In contrast, many cancer cells harbor defects in key DNA repair pathways, rendering them particularly vulnerable to chemotherapy-induced genotoxic stress. Tumors with BRCA1 or BRCA2 mutations, for example, lack efficient homologous recombination repair, making them exquisitely sensitive to DNA crosslinking agents and PARP inhibitors. In these cases, DNA damage becomes synthetically lethal, leading to apoptosis or mitotic catastrophe. This principle has been therapeutically exploited in the context of “BRCAness,” where cancers with homologous recombination deficiency respond preferentially to DNA-damaging agents.
However, not all tumors are equally deficient in repair mechanisms. Some retain partial or adaptive repair functions that allow for survival in the face of ongoing DNA insult. Through selection pressure, these cells may upregulate compensatory pathways, such as enhanced activity of PARP enzymes or increased expression of error-prone repair mechanisms, contributing to chemoresistance. Moreover, mutator phenotypes, often driven by mismatch repair deficiency or APOBEC activity, can generate genomic heterogeneity that facilitates the emergence of resistant clones under treatment pressure.
On the other side of the therapeutic equation, normal cells that survive DNA damage may accumulate mutations over time, particularly if repair is imperfect or overwhelmed by repeated injury. This can predispose patients to secondary malignancies, a well-recognized late complication of chemotherapy—most notably, therapy-related acute myeloid leukemia (t-AML) following alkylating agents or topoisomerase II inhibitors.
Chemotherapy-Induced Senescence and Immune Modulation
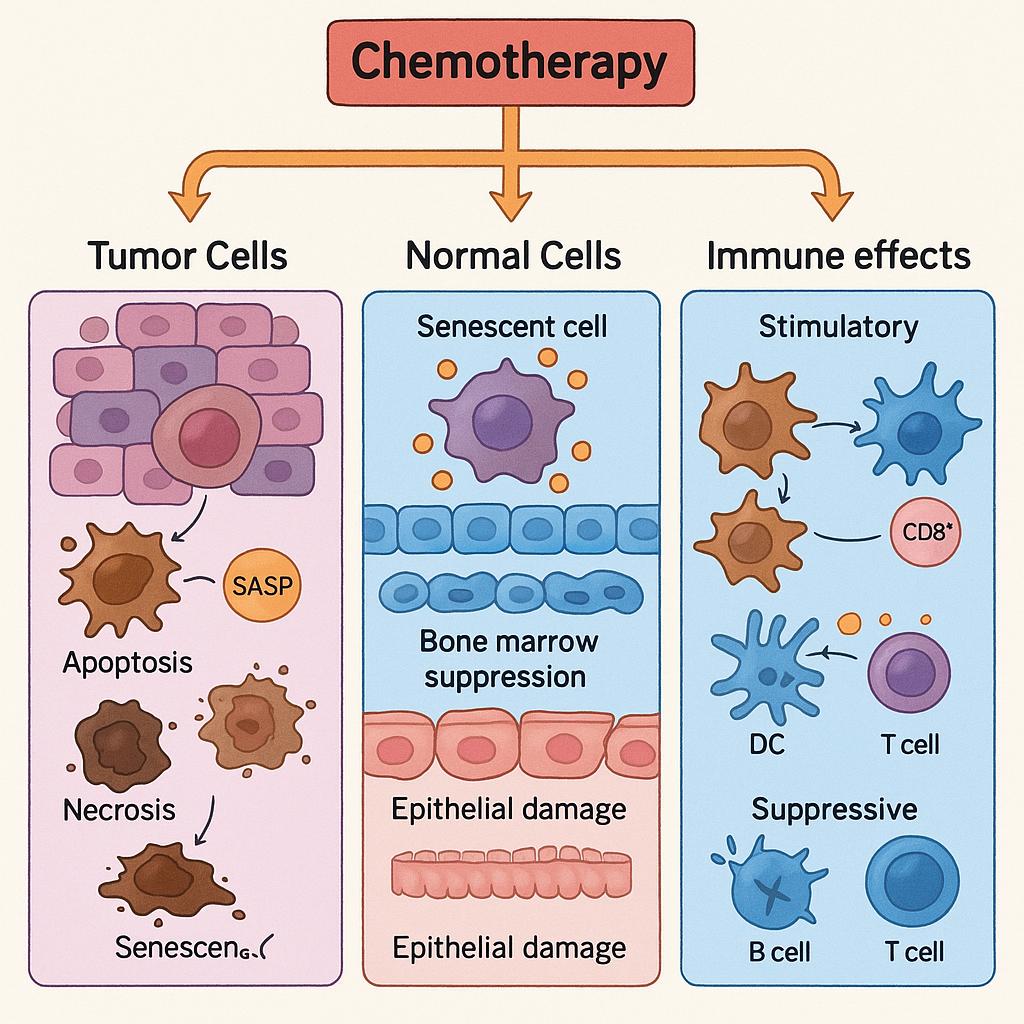
Beyond apoptosis and necrosis, a less overt but biologically significant outcome of chemotherapy is the induction of cellular senescence—a state of permanent cell cycle arrest without immediate cell death. Chemotherapy-induced senescence (CIS) can occur in both cancer cells and normal cells as a consequence of sublethal DNA damage, oxidative stress, or oncogene activation. While senescent cells do not proliferate, they remain metabolically active and secrete a diverse array of bioactive molecules collectively known as the senescence-associated secretory phenotype (SASP), which includes pro-inflammatory cytokines, chemokines, growth factors, and matrix-modifying enzymes.
In the context of tumor cells, senescence may initially function as a tumor-suppressive mechanism, halting the growth of damaged or transformed cells and preventing further propagation of genomic instability. Indeed, some chemotherapeutic regimens intentionally aim to induce senescence as a non-lethal means of disease control. However, the persistence of senescent cancer cells may paradoxically contribute to therapy resistance and tumor relapse, particularly if cells escape from the arrested state or if the SASP fosters a supportive microenvironment for tumor regrowth.
In normal tissues, senescence is more clearly detrimental over time. Senescent non-malignant cells accumulate following chemotherapy exposure, especially in tissues with high proliferative demands such as bone marrow and mucosal surfaces. The chronic release of SASP factors by these cells creates a pro-inflammatory milieu, which can impair tissue regeneration, exacerbate fibrosis, and promote age-related pathologies. This persistent, low-grade inflammation may also contribute to chemotherapy-induced frailty, cardiovascular dysfunction, and impaired organ function in long-term survivors.
Chemotherapy also exerts profound effects on the immune system, with both stimulatory and suppressive consequences. On one hand, certain chemotherapeutic agents induce immunogenic cell death (ICD) in tumor cells, characterized by the release of damage-associated molecular patterns (DAMPs) such as calreticulin, HMGB1, and ATP. These molecules enhance antigen presentation, recruit dendritic cells, and stimulate cytotoxic T cell responses, thereby linking chemotherapy to anti-tumor immunity. This phenomenon has laid the groundwork for combining cytotoxic agents with immune checkpoint inhibitors, aiming to transform immunologically “cold” tumors into “hot” ones.
On the other hand, chemotherapy can be deeply immunosuppressive. Many agents indiscriminately deplete rapidly dividing immune cells, leading to lymphopenia, myelosuppression, and compromised host defense. This not only increases susceptibility to infections but may also impair the long-term capacity for tumor immune surveillance. The timing, dose intensity, and type of chemotherapeutic agent largely determine the balance between immune activation and suppression.
Strategies to Minimize Chemotherapy Toxicity to Normal Tissues
Given the systemic nature and limited selectivity of conventional chemotherapy, a major focus of contemporary oncology has been the development of strategies to reduce off-target toxicity without compromising anticancer efficacy. These efforts encompass both therapeutic innovations and supportive care interventions, aiming to widen the therapeutic index and tailor treatment to individual patient profiles.
One of the foundational approaches is dose optimization and scheduling modification. Traditional chemotherapy regimens are often administered in high doses at spaced intervals to maximize tumor cell kill. However, alternative strategies such as metronomic chemotherapy—the continuous or frequent administration of low-dose chemotherapy—aim to reduce toxicity while targeting tumor angiogenesis and minimizing immunosuppression. This approach has shown promise in certain solid tumors and is being actively investigated in combination with immunotherapy.
Targeted drug delivery technologies offer another avenue to enhance selectivity. Liposomal formulations, such as liposomal doxorubicin, encapsulate chemotherapeutic agents in lipid vesicles that preferentially accumulate in tumors via enhanced permeability and retention (EPR) effects, thereby reducing systemic exposure and associated toxicities. Similarly, antibody-drug conjugates (ADCs) represent a rapidly expanding class of therapeutics that combine the specificity of monoclonal antibodies with the potency of cytotoxic agents. By directing chemotherapy to cells expressing a specific surface antigen, ADCs deliver high concentrations of drug to tumor cells while sparing healthy tissue.
To counteract organ-specific toxicity, chemoprotective agents have been developed. For example, dexrazoxane is used to mitigate anthracycline-induced cardiotoxicity by chelating iron and reducing oxidative stress in cardiac tissue. Other agents, such as amifostine and mesna, protect against nephrotoxicity and hemorrhagic cystitis, respectively, without diminishing the efficacy of chemotherapy.
In addition, supportive therapies play a critical role in minimizing treatment-related complications and maintaining dose intensity. Granulocyte colony-stimulating factors (G-CSF) are routinely used to reduce the duration and severity of chemotherapy-induced neutropenia, thereby lowering infection risk. Antiemetics, including 5-HT3 receptor antagonists and NK1 inhibitors, have substantially improved the management of chemotherapy-induced nausea and vomiting, enhancing treatment adherence and patient comfort.
A major frontier in toxicity mitigation lies in the application of biomarkers of susceptibility, enabled by advances in pharmacogenomics. Testing for DPYD (dihydropyrimidine dehydrogenase) gene variants is now recommended before administering fluoropyrimidines like 5-fluorouracil or capecitabine, as deficiencies in this enzyme can lead to life-threatening toxicity. Similar genetic screening tools are being explored for TPMT (thiopurine methyltransferase), UGT1A1, and CYP2D6, guiding drug selection and dosing for a variety of agents.
These strategies converge under the umbrella of precision oncology, which seeks to individualize therapy based on a combination of tumor profiling, pharmacogenetic testing, and host characteristics. By tailoring chemotherapy to the molecular and physiologic features of both the cancer and the patient, modern treatment paradigms aim to maximize therapeutic gain while minimizing harm—a shift that holds the promise of transforming cytotoxic chemotherapy into a more personalized and tolerable component of cancer care.
Resistance and the Tumor–Host Paradox
A key paradox in chemotherapy lies in its differential impact on cancer versus normal cells over time. While normal tissues often lack the ability to adapt and are progressively damaged by cumulative toxicity, cancer cells can evolve resistance mechanisms that allow them to survive and proliferate despite ongoing treatment. These adaptations include enhanced DNA repair, upregulation of drug efflux pumps, and metabolic reprogramming. Moreover, chemotherapy can unintentionally select for resistant subclones or cancer stem-like cells, which are more quiescent and inherently less sensitive to cytotoxic agents. As a result, treatment may initially reduce tumor bulk but ultimately enrich for a more aggressive, therapy-resistant population. In contrast, normal cells, particularly those with high turnover, continue to suffer irreversible damage, contributing to chronic side effects and long-term complications in cancer survivors. This imbalance underscores the urgent need for more selective and adaptive treatment approaches that can overcome tumor resistance without compromising host tissue integrity.
Long-Term Toxicities and Survivorship Challenges
While chemotherapy can be life-saving, its impact on normal tissues may result in chronic, and sometimes irreversible, complications. Secondary malignancies, particularly therapy-related leukemias, can emerge years after treatment due to chemotherapy’s mutagenic effects on hematopoietic stem cells. Fertility impairment is a common concern, especially in younger patients, as cytotoxic agents can damage gonadal tissue and lead to premature ovarian failure or testicular dysfunction. Cognitive changes, often described as “chemo brain,” include memory lapses, attention deficits, and executive dysfunction, which can persist long after treatment. Additionally, organ-specific damage—such as cardiomyopathy, renal impairment, or pulmonary fibrosis—may limit long-term health and quality of life.
These risks highlight the importance of comprehensive survivorship care plans that monitor for late effects, address functional and psychosocial needs, and guide follow-up interventions. For patients with curable cancers, particularly children and young adults, treatment decisions must carefully balance curative intent with the potential for lasting harm, emphasizing the need for risk-adapted, less toxic regimens when possible.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
How does chemotherapy kill cancer cells?
Chemotherapy targets rapidly dividing cells by disrupting DNA synthesis, mitosis, or cellular metabolism, ultimately leading to cell death through apoptosis or mitotic catastrophe.
Why does chemotherapy affect healthy cells too?
Many healthy tissues, like bone marrow and gut lining, also divide rapidly, making them vulnerable to the same mechanisms that target cancer cells.
What are the most common side effects of chemotherapy?
Side effects include nausea, hair loss, neutropenia, anemia, mucositis, and peripheral neuropathy, depending on the drug and dosage.
Can chemotherapy cause long-term damage?
Yes. It can lead to secondary cancers, infertility, organ damage, and cognitive changes, particularly in long-term survivors.
How is chemotherapy toxicity minimized?
Through supportive care (like G-CSF, antiemetics), precision dosing, liposomal or targeted delivery systems, and genetic screening to personalize treatment.
What is chemotherapy-induced senescence?
It’s a state where cells stop dividing but don’t die, which can suppress tumor growth or, paradoxically, contribute to relapse and inflammation.
Can chemotherapy enhance the immune response to cancer?
Yes. Some drugs induce immunogenic cell death, releasing signals that activate immune responses and synergize with immunotherapy.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
