At OncoDaily GI, we spotlight the breakthroughs redefining gastrointestinal cancer care — from molecular precision to surgical innovation and translational discovery. As the first week of October uncovers major milestones across Europe and the U.S., Italy’s national genomics platform reaches a landmark moment, new strategies emerge in liver surgery and transplantation, and teams at MSK and MD Anderson unveil advances in pancreatic and biliary cancers.
From triplet chemotherapy before hepatectomy to engineered exosomes targeting KRAS, liquid biopsy and biomarker-driven therapies, and AI-guided radiotherapy, these updates showcase how global collaboration is transforming GI oncology.
Giuseppe Curigliano, MD, PhD – ESMO President-Elect; Professor of Medical Oncology, University of Milan
“The ROME trial marks a milestone for Italy’s precision oncology platform: 6 years of dedication, 40 centers, 1,794 patients sequenced. A national effort to bring genomics to the heart of cancer care.”

Read more about ROME Trial: Genomically Matched Therapy in Advanced Solid Tumors on OncoDaily.

Elena Panettieri, MD, PhD – Università Cattolica del Sacro Cuore, Rome; European-African Hepato-Pancreato-Biliary Association (E-AHPBA)
“Triplet chemo before liver surgery: risk or reward?
Our new multicenter study (n=1,711, 3 international centers) compared FOLFOXIRI (triplet) vs. FOLFOX/FOLFIRI (doublets) before hepatectomy for colorectal liver metastases (CLM).
What we found
Short-term outcomes: No increase in major complications or 90-day mortality with FOLFOXIRI ✅.
Long-term outcomes: Overall survival similar across groups — but in patients with medium–high tumor burden, FOLFOXIRI significantly improved 5-year OS (67.6% vs. 50.1%, p=0.004).
Multivariable analysis: In the medium–high tumor burden subgroup, FOLFOXIRI was independently associated with improved survival (HR 0.56).
Key message: In select patients with more aggressive disease, FOLFOXIRI before surgery is safe — and may unlock a real survival advantage in those with higher tumor burden.”
Heithem Jeddou, MD – HPB and Liver Transplant Surgery, CHU de Rennes
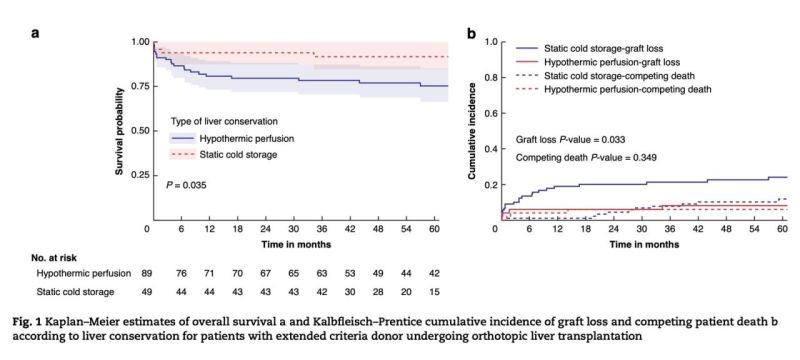
“Proud to share the results of Agathe Coquelle thesis, co-supervised by Stylianos Tzedakis and myself, on hypothermic oxygenated machine perfusion (HOPE) for brain dead (DBD) extended-criteria donor (ECD) liver transplantation recently published on British Journal of Surgery
Key question: Dynamic preservation with HOPE has shown significant reductions in early allograft dysfunction, liver-related and biliary complications but what about long-term outcomes ?
Design: HOPE cases; 1:2 propensity-matched comparison vs static cold storage (SCS).
2018–2021 cohort of 49 HOPE vs 89 SCS with a median follow-up of 62.5 (95%CI: 35.0 – 124.4) months.
Key findings supporting HOPE:
-> Death-censored graft survival was higher at 1, 3 and 5 years (91.7% versus 75.2%, log-rank p=0.035)
-> Lower cumulative graft loss with or without death-competing risks (8.1% vs 22.6%, Gray p=0.033)
-> Independent effect on graft survival (Cox adjusted-HR: 0.30, 95%CI 0.10–0.91, p=0.045; Fine-Gray adjusted-sHR: 0.38, 95%CI 0.16–0.58, p=0.046)
-> Fewer non-anastomotic biliary complications (8.2% vs 24.7%, p=0.022) and severe postoperative complications (30.8% vs 52.7%, p=0.030)
-> Shorter hospital stay (median 15 vs 20 days, p=0.018)Key message: As ECD use grows, HOPE offers a pragmatic, scalable way to improve long-term graft survival beyond early outcomes.”
Simonetta Maria Leto, PhD – Senior Research Associate, Candiolo Cancer Institute – FPO IRCCS, Turin
“Proud moment! Our paper has been published in Cancer Discovery!
I’m excited to share how our amazing team solved a critical puzzle in colorectal cancer treatment. We discovered why FOLFIRI chemotherapy works for some patients but not others, identifying new biomarkers that could help clinicians predict treatment response and select optimal therapies for each patient.
This work opens new avenues for precision medicine in colorectal cancer treatment. I’m so proud to be part of this collaborative effort!”

Felipe Couñago, MD, PhD – Medical Director, GenesisCare Spain; Radiation Oncologist and Clinical Researcher
“Proud to share the first Spanish prospective study on MR-Linac for pancreatic cancer just published in Biomedicines!
28 patients with BRPC/LAPC treated with SMART (5×40–50 Gy) on 0.35T MR-Linac
100% daily online adaptation, safe & feasible
0% ≥G3 toxicity
Outcomes: LC 89% @6m, OS 74% @12m, PFS median 11.5m (65.6% @12m)
This milestone highlights the strength of our research at GenesisCare España and the dedication of an amazing team.
A very special to Daniela Gonsalves Pieretti , as this marks the final work of her PhD thesis – what an achievement!
Congratulations to all authors & collaborators for making history in Spanish MRgRT for pancreas.”

Jannis Duhn, MD – General Surgery Resident, University Hospital Schleswig-Holstein (UKSH), Lübeck
“In our latest publication in Langenbeck‘s Archives of Surgery, we show that Pancreatic Colloid Carcinomas are in comparison to Ductal Adenocarcinomas:
Diagnosed at earlier tumor stages
Independently associated with improved overall survival
Associated with improved survival following adjuvant chemotherapy in advanced stages only.
This study proved again the power of nationwide registry based analyses, enabled by the Arbeitsgemeinschaft Deutscher Tumorzentren e.V. (ADT-Netzwerk).”
Raghu Kalluri, MD, PhD – Professor and Chairman, Department of Cancer Biology, The University of Texas MD Anderson Cancer Center
“It’s Official! Our Phase 1 clinical trial of iExosomes in metastatic pancreatic cancer is now published in Nature Communications.
Zero DLTs in this first-in-human trial of systemically administered engineered exosomes targeting KRAS G12D!!
This was a truly heroic team effort by more than 90 colleagues across 17 departments, cores, and offices…start to finish….including non-human primate studies and manufacturing of clinical GMP-grade drug. Just 5 years from discovery in Kalluri Lab -> patients, and only possible at MD Anderson Cancer Center.”
Read more about iEXPLORE Phase I Trial: Engineered Exosomes with KRASG12D siRNA in Pancreatic Cancer on OncoDaily.
Andrea Wang-Gillam, MD, PhD – Oncologist and Drug Developer
“Excited to share our new publication in Cancer Communication on glecirasib (KRAS G12C inhibitor).
The study demonstrated promising clinical benefit in PDAC, BTC and other KRAS G12C-mutant tumors, where treatment options remain limited.
Grateful to patients across regions who participated in these studies!
Congratulations to my co-authors for their dedication and insight.”
Marc Hilmi, MD, PhD – GI Oncology Research Scholar, Center for Pancreatic Cancer Research, Memorial Sloan Kettering Cancer Center
“Exciting milestone after almost one year at Memorial Sloan Kettering Cancer Center in the Center for Pancreatic Cancer Research
I had the privilege to present at AACR Pancreas in Boston our translational work on the POLAR trial, where we identified key mechanisms of resistance and response to treatment (with impressive HR < 0.10) through integrated RNA and TCR single-cell sequencing.
This progress was only possible thanks to fantastic collaborations with Vinod Balachandran, Benjamin Greenbaum, Dana Pe’er, and Mara Sherman.
Looking forward to an exciting second year!”

James J. Harding, MD – Associate Attending, Gastrointestinal Oncology and Early Drug Development Services, Memorial Sloan Kettering Cancer Center; Associate Professor of Medicine, Weill Cornell Medical College
“Thank you AACR Journals for publishing our manuscript on a novel, dual, IDH1/2 inhibitor for biliary tract cancer and other rare cancers on rare cancer day! Important learnings and hypothesis generating data from Memorial Sloan Kettering Cancer Center. Global collaboration at its best with Masafumi Ikeda Rachna Shroff, MD, FASCO Antoine Hollebecque Mitesh Borad, M.D. Anthony El-khoueiry Jordi Rodón and many others. Appreciate”

You can read about the 10 Must-Read Posts in GI Oncology from the last week of September on OncoDaily.


