iEXPLORE Phase I trial (NCT03608631) marks the study of engineered exosomes carrying KRASG12D-specific siRNA (iExoKRASG12D) for patients with advanced pancreatic ductal adenocarcinoma (PDAC). Given that over 90% of PDAC tumors harbor KRAS mutations—half of them with the KRASG12D variant—this pathway represents one of the most critical therapeutic targets in pancreatic cancer.
Historically considered “undruggable,” KRAS has resisted conventional small molecule inhibition, leaving patients with few effective treatment options beyond chemotherapy. iExoKRASG12D offers a novel precision approach by using exosomes as natural delivery vehicles to selectively silence oncogenic KRAS, reduce downstream signaling, and remodel the tumor microenvironment. The iEXPLORE trial was designed to evaluate safety, tolerability, and biological activity of this therapy, while also providing mechanistic insights into its potential role in combination strategies with immune checkpoint inhibitors.
Title: Engineered exosomes with KrasG12D specific siRNA in pancreatic cancer: a phase I study with immunological correlates
Authors: Valerie S. Kalluri, Brandon G. Smaglo, Krishnan K. Mahadevan, Michelle L. Kirtley, Kathleen M. McAndrews, Mayela Mendt, Sujuan Yang, Ana S. Maldonado, Hikaru Sugimoto, Maria E. Salvatierra, Luisa M. Solis Soto, Cara Haymaker, Rick Finch, Mihai Gagea, Adam C. Fluty, Steven J. Ludtke, J. Jack Lee, Abhinav K. Jain, Gauri Varadhachary, Rachna T. Shroff, Anirban Maitra, Elizabeth Shpall, Shubham Pant & Raghu Kalluri.
Background
Pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest cancers, with a median survival of less than 12 months despite combination chemotherapy. More than 90% of PDAC tumors harbor oncogenic KRAS mutations, with KRASG12D found in about half of cases. Targeting KRAS has long been considered “undruggable,” but new strategies—including small molecule inhibitors and RNA-based approaches—have renewed optimism.
Engineered exosomes, or extracellular vesicles, offer a promising delivery vehicle for KRAS-directed therapies due to their natural biodistribution to the pancreas and reduced immunogenicity. The iEXPLORE Phase I trial (NCT03608631) evaluated the safety, tolerability, and biological activity of engineered exosomes loaded with KRASG12D-specific siRNA (iExoKRASG12D) in patients with advanced, treatment-refractory PDAC.
Methods
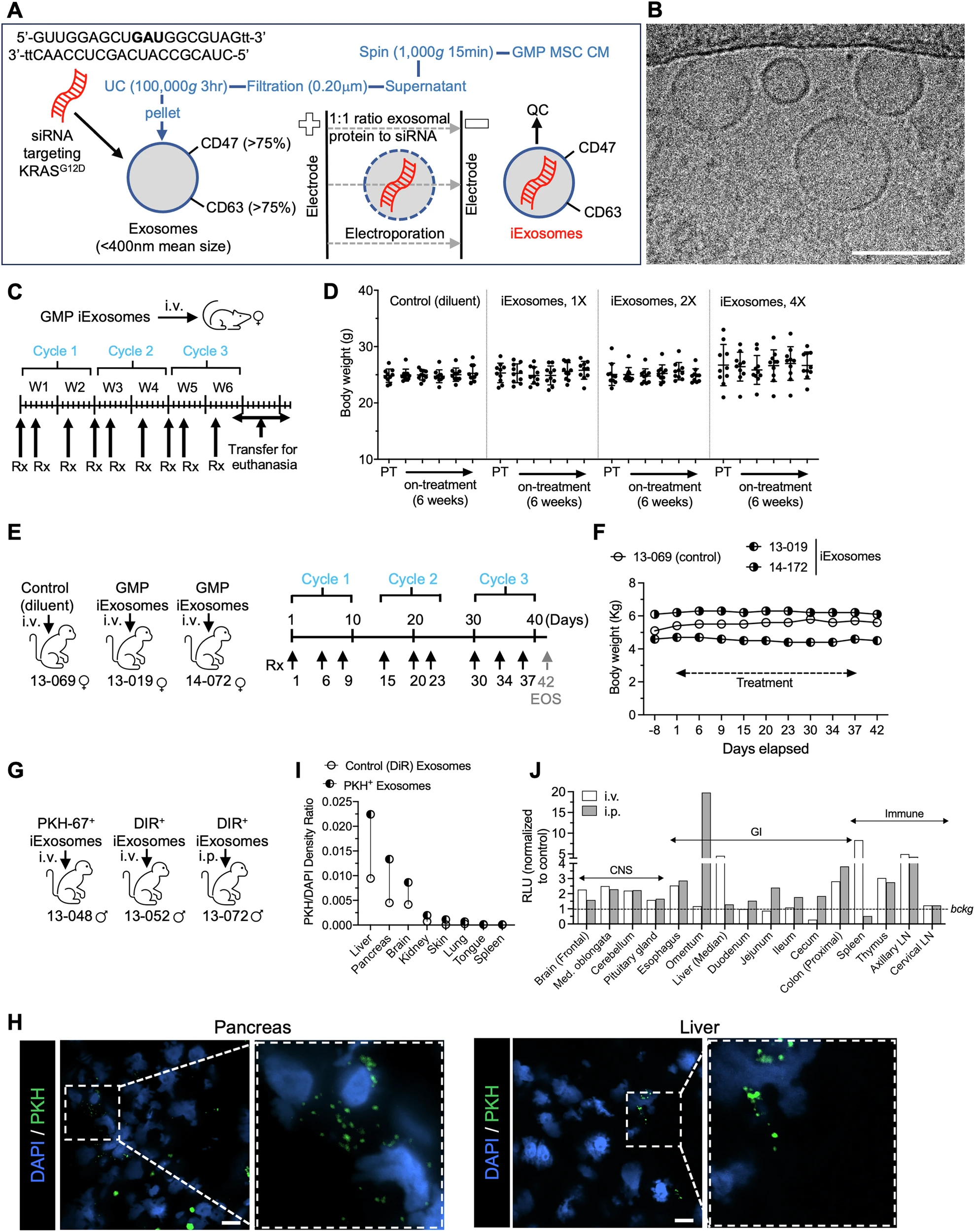
Preclinical testing was first performed in mice and rhesus macaques. Exosomes were derived from bone marrow mesenchymal stromal cells (MSCs), electroporated with siRNA targeting KRASG12D, and produced under Good Manufacturing Practice (GMP) conditions. Toxicology and biodistribution studies established safety and demonstrated preferential accumulation in the pancreas and liver.
For the clinical trial, patients were eligible if they had histologically confirmed metastatic PDAC harboring KRASG12D mutation, progression after at least one prior systemic therapy, and ECOG performance status 0–1. The study evaluated escalating doses of iExoKRASG12D, delivered intravenously, with primary endpoints of safety and tolerability. Secondary endpoints included evidence of target engagement, changes in circulating tumor DNA (ctDNA), and disease control.
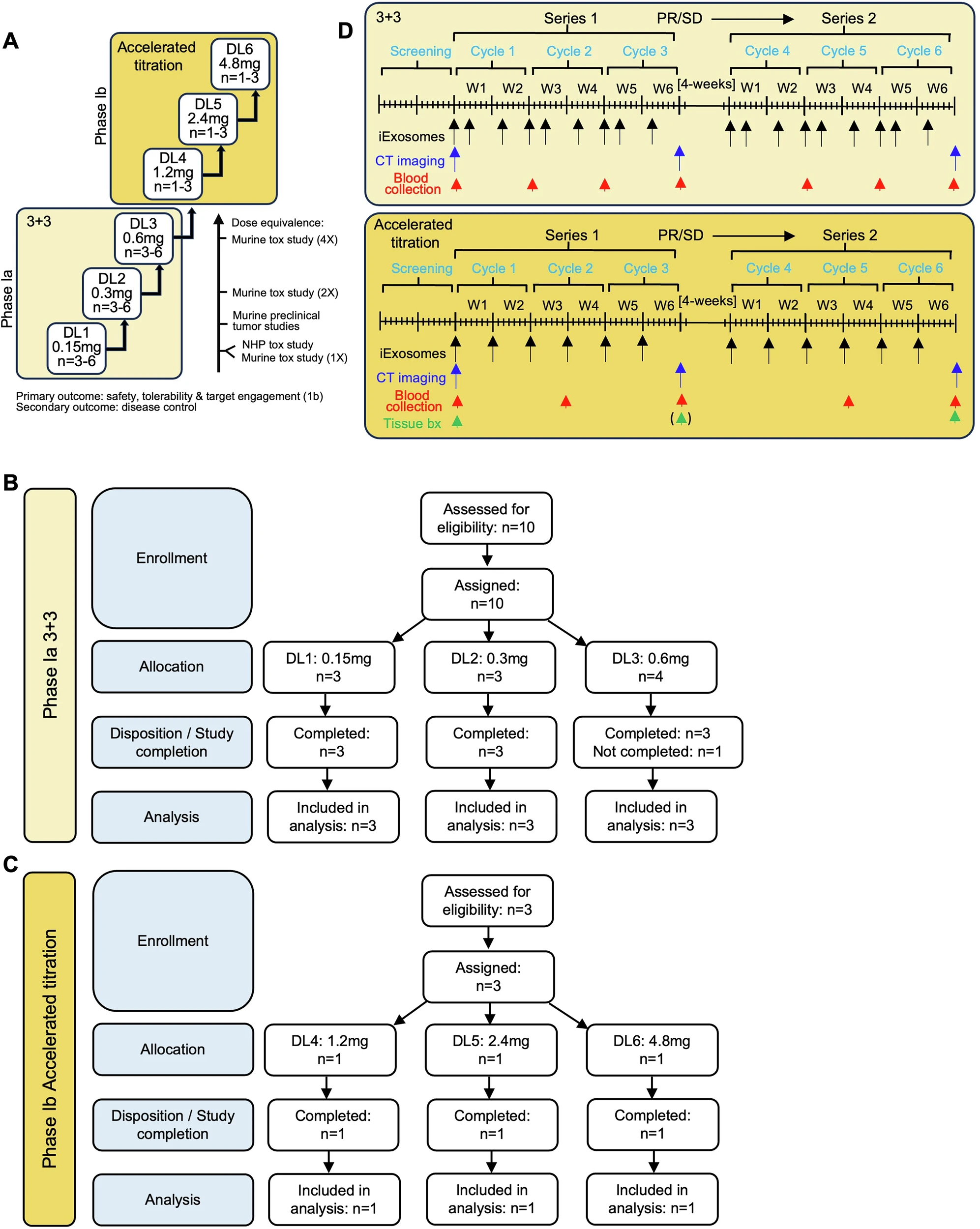
Study Design
The iEXPLORE study consisted of two parts.
- Phase Ia: A classical 3+3 dose-escalation enrolled 10 evaluable patients across three dose levels. Patients received three doses of iExoKRASG12D over two weeks (one cycle), repeated for three cycles.
- Phase Ib: An accelerated titration design enrolled three patients at higher dose levels, with weekly infusions for six weeks. Tissue biopsies were obtained before and after therapy.
Preclinical models were also used to explore combination strategies with immune checkpoint blockade, given prior observations of CD8+ T cell recruitment after KRAS suppression.
Results
Preclinical findings:
- In mice, repeated iExoKRASG12D dosing (up to 9 doses over 6 weeks) showed no changes in body weight, liver or kidney function, or hematology.
- In rhesus macaques, nine doses were also well tolerated, with no histopathological abnormalities. Biodistribution confirmed siRNA delivery to the pancreas, liver, and bone marrow.
Phase Ia clinical trial (n=10 evaluable patients, age 49–75 years):
- No dose-limiting toxicities (DLTs) were observed at any dose level.
- Maximum tolerated dose (MTD) was not reached, even at the highest dose of 4.8 mg siRNA per infusion.
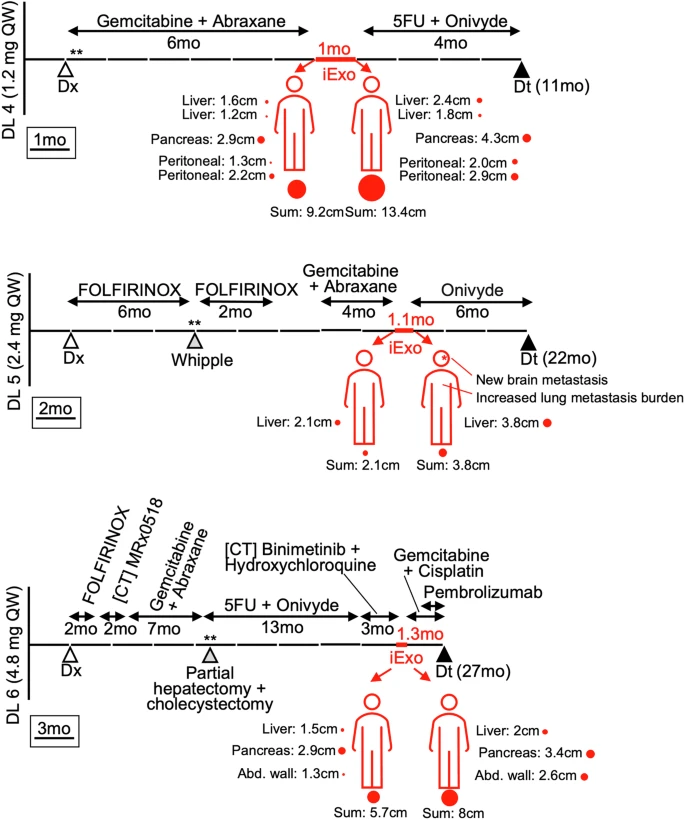
- Three patients achieved stable disease after three cycles, one maintaining stability for up to six cycles before progression.All others eventually progressed, with time from diagnosis to death ranging 1–7 years.
Phase Ib accelerated titration (n=3 patients, age 48–76 years):
- All three achieved stable disease during treatment before progressing.
- No treatment-related adverse events were reported.
- Biopsies demonstrated downregulation of phosphorylated ERK, reduction in PanCK+ tumor cells, and increased intratumoral CD8+ T cells post-treatment.
Circulating tumor DNA (ctDNA) analysis:
- Several patients showed reduced KRASG12D mutant allele fraction after treatment, consistent with target engagement.
Combination studies:
- Preclinical models showed that iExoKRASG12D increased Fas receptor expression on tumor cells, enabling CD8+ T cell–mediated apoptosis.
- Combination of iExoKRASG12D with anti-CTLA-4 antibodies produced significant tumor regression, increased tertiary lymphoid structures (TLS), and robust survival benefit in mouse models.
- No such synergy was observed with anti-PD-1 therapy.
Key Findings
- iExoKRASG12D was well tolerated in both animal models and humans, with no dose-limiting toxicities or immune-related adverse events.
- Target engagement: Evidence of KRASG12D downregulation, ERK suppression, and reduced ctDNA confirmed biological activity.
- Treatment led to increased intratumoral CD8+ T cell infiltration and favorable remodeling of the tumor microenvironment.
- While no objective responses were seen, several patients achieved temporary stable disease, a meaningful outcome in heavily pretreated metastatic PDAC.
- Preclinical data strongly support combination with CTLA-4 blockade as a next step toward clinical efficacy.
Key Takeaway Messages
The iEXPLORE trial provides the first-in-human evidence that engineered exosomes can safely deliver siRNA against KRASG12D in advanced PDAC. Although monotherapy produced disease stabilization rather than tumor shrinkage, consistent biological activity and immune priming suggest that iExoKRASG12D could serve as a foundation for rational combination strategies. In particular, synergy with anti-CTLA-4 therapy offers a promising path forward, potentially overcoming the resistance of PDAC to checkpoint blockade.
Conclusion
This pioneering Phase I study demonstrates the feasibility and safety of exosome-based delivery of KRASG12D-targeted siRNA in pancreatic cancer patients. The absence of toxicity, evidence of target engagement, and remodeling of the tumor immune landscape represent a critical milestone in efforts to drug “undruggable” oncogenes. While monotherapy yielded stable disease in select patients, the integration of iExoKRASG12D with immunotherapies such as anti-CTLA-4 antibodies appears to unlock greater antitumor efficacy in preclinical models. These findings lay the groundwork for Phase II trials and highlight exosome therapeutics as a novel precision medicine approach for one of the most treatment-resistant cancers.
You can read the full article here.