The ROME trial represents a landmark effort in precision oncology, providing the first randomized phase 2 evidence that genomically matched therapies guided by a molecular tumor board can improve outcomes in patients with advanced solid tumors.
Conducted across multiple Italian cancer centers, the study compared tailored treatment based on genomic profiling with standard of care in heavily pretreated patients. By integrating next-generation sequencing with multidisciplinary decision-making, the trial not only demonstrated higher response rates and longer progression-free survival but also highlighted the practical role of precision oncology in routine clinical practice.
Title: Genomically matched therapy in advanced solid tumors: the randomized phase 2 ROME trial
Authors: Paolo Marchetti, Giuseppe Curigliano, Mauro Biffoni, Sara Lonardi, Simone Scagnoli, Lorenzo Fornaro, Valentina Guarneri, Ugo De Giorgi, Paolo Antonio Ascierto, Giovanni Blandino, Giulia D’Amati, Massimo Aglietta, Chiara Cremolini, Pierfranco Conte, Edoardo Crimini, Maurizio Ceracchi, Simona Pisegna, Sofia Verkhovskaia, Roberto Bordonaro, Sergio Bracarda, Giovanni Butturini, Lucia Del Mastro, Andrea DeCensi, Agnese Fabbri, Elisabetta Fenocchio, Stefania Gori, Giulio Metro, Annamaria Pessino, Daniele Pozzessere, Fabio Puglisi, Stefano Tamberi, Alberto Zambelli, Donatella Marino, Ettore Capoluongo, Federico Cappuzzo, Bruna Cerbelli, Giuseppe Giannini, Umberto Malapelle, Federica Mazzuca, Marianna Nuti, Giancarlo Pruneri, Maurizio Simmaco, Lidia Strigari, Giuseppe Tonini, Nello Martini & Andrea Botticelli.
Published in Nature Medicine, September 2025
Background
Precision oncology is built on the principle that identifying genomic alterations within tumors can guide more effective, individualized treatment. Several tumor-agnostic biomarkers, such as microsatellite instability-high (MSI-H), high tumor mutational burden (TMB), NTRK fusions, BRAF V600E mutations, RET fusions, and HER2 alterations, have already demonstrated the potential to drive therapeutic benefit across different cancers.
However, randomized evidence directly comparing genomically matched therapies against standard of care (SoC) in advanced solid tumors has been limited. The randomized phase 2 ROME trial was designed to evaluate whether tailored treatment (TT) based on molecular profiling and guided by a molecular tumor board (MTB) improves outcomes in patients with advanced solid tumors after one or two prior lines of therapy.
Methods
Between October 2020 and July 2023, 1,794 patients with metastatic solid tumors and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 were screened at 40 oncology centers across Italy. Genomic testing was performed using FoundationOne CDx (tissue) and FoundationOne Liquid CDx (blood) to identify actionable molecular alterations.
Of these, 897 patients with potentially actionable alterations were evaluated by an MTB. The MTB included oncologists, pathologists, geneticists, bioinformaticians, and other specialists, and made treatment recommendations based on genomic data and clinical context. Patients with suitable targets were randomized in a 1:1 ratio to either TT or SoC.
Study Design
This multicenter, open-label phase 2 trial randomized 400 patients in a 1:1 ratio to tailored treatment (TT) or standard of care (SoC).Treatment arms wew
- TT arm: Patients received targeted therapies, immunotherapies, or combinations as determined by the MTB.
- SoC arm: Patients received chemotherapy, immunotherapy, or targeted therapy according to tumor type and national guidelines.
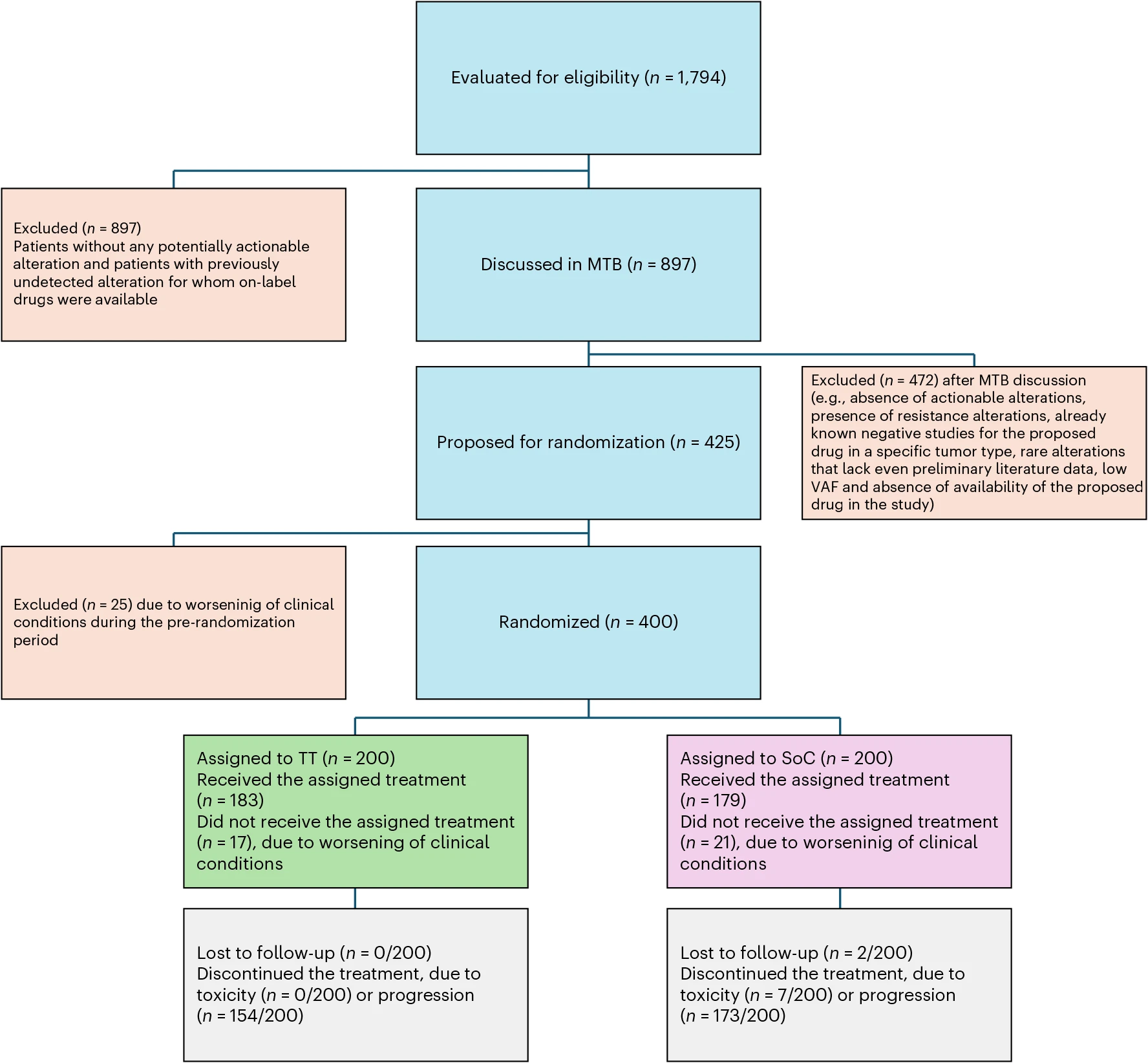
Outcomes were assessed across all randomized patients, with efficacy evaluated by overall response rate (ORR) as the primary endpoint and progression-free survival (PFS), overall survival (OS), and additional measures as secondary endpoints. The analysis revealed meaningful differences between TT and SoC, particularly in tumor response and disease control, while also providing insight into subgroup benefits and the impact of crossover on survival outcomes.
Results
The efficacy and safety outcomes from the ROME trial demonstrated clear advantages for tailored treatment over standard of care, with notable improvements in response rates and disease control across multiple tumor types.
Overall response rate (ORR):
- TT: 17.5% (95% CI 12.5–23.5), including 3% complete responses and 14.5% partial responses.
- SoC: 10.0% (95% CI 6.2–15.0), all partial responses.
- P = 0.0294.
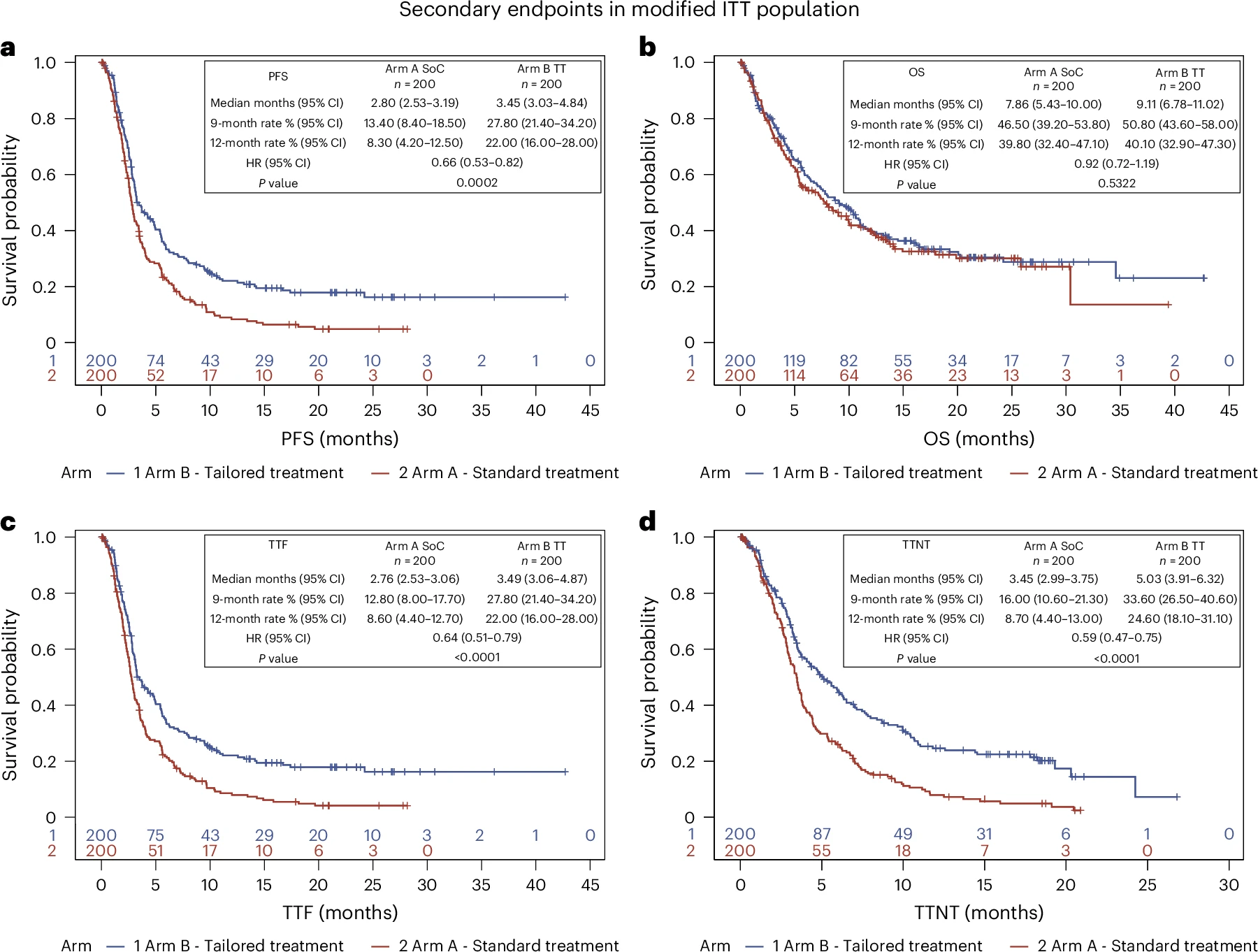
Progression-free survival (PFS):
- Median PFS: 3.5 months (TT) vs 2.8 months (SoC).
- Hazard ratio (HR): 0.66 (95% CI 0.53–0.82; P = 0.0002).
- 12-month PFS: 22.0% (TT) vs 8.3% (SoC).
Overall survival (OS):
- Median OS: 9.1 months (TT) vs 7.9 months (SoC).
- HR: 0.92 (95% CI 0.72–1.19; P = 0.53).
- High crossover rate (~59%) from SoC to TT likely diluted OS differences.
Other secondary outcomes:
- Median TTF: 3.5 months (TT) vs 2.8 months (SoC) (HR = 0.64, P < 0.0001).
- Median TTNT: 5.0 months (TT) vs 3.5 months (SoC) (HR = 0.59, P < 0.0001).
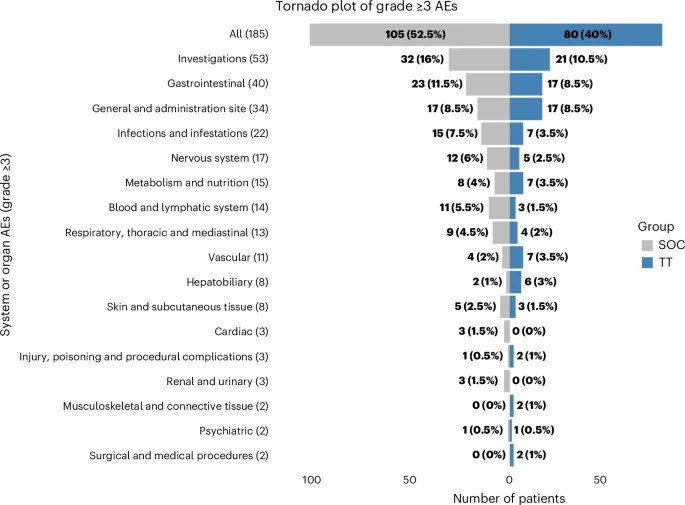
Safety:
- Grade ≥3 adverse events (AEs): 40% (TT) vs 52.5% (SoC).
- TT arm most common AE: diarrhea (5.5%).
- SoC arm most common AE: neutropenia (8%).
Key Findings
- Beyond the primary efficacy outcomes, several important insights emerged from subgroup analyses and safety evaluations, further underscoring the clinical relevance of genomically guided therapy.
- TT significantly improved ORR and PFS compared to SoC across multiple tumor types.
- Durable control: At 12 months, 22% of TT patients remained progression-free compared to only 8% with SoC.
- OS was not improved, largely due to high crossover from SoC to TT.
Exploratory subgroup analyses:
- MSI-H (n=18): ORR 57% (TT) vs 0% (SoC); median PFS not reached in TT arm.
- High TMB (n=135): ORR 25.7% (TT) vs 15.4% (SoC); HR for PFS = 0.64.
- BRAF-altered tumors (n=14): PFS not reached with TT vs 2.0 months with SoC (HR = 0.07).
- HER2-altered tumors (n=58): Median PFS 4.6 months (TT) vs 2.6 months (SoC); HR = 0.40.
The MTB framework proved essential, ensuring clinically relevant decision-making in the presence of multiple actionable targets.
Key Takeaway Messages
- Precision oncology works: Matching therapies to molecular profiles improved both response rates and disease control.
- Crossover affects OS readouts: While survival differences were not seen, cross-treatment exposure complicates interpretation.
- MTBs are critical: They provide multidisciplinary expertise that enables rational therapy selection beyond simple mutation–drug matching.
- Subgroups matter: Patients with MSI-H, high TMB, BRAF, and HER2 alterations benefited the most.
- Safety remained acceptable, with fewer high-grade AEs in the TT arm compared with SoC.
Conclusion
The ROME trial provides robust randomized evidence supporting the integration of comprehensive genomic profiling and molecular tumor board-driven decisions into clinical oncology practice. Patients receiving genomically matched tailored therapies demonstrated significantly improved response rates and progression-free survival compared to standard of care, with consistent benefits across key biomarker subgroups. While OS benefit was not observed, mainly due to crossover, the results highlight the importance of early genomic testing and the deployment of targeted treatments at earlier stages of metastatic disease.
The trial establishes that tumor-agnostic precision oncology strategies can meaningfully extend disease control, especially when guided by multidisciplinary expertise. These findings reinforce the need for broader adoption of genomic profiling and structured MTB integration to optimize outcomes in advanced solid tumors.
You can read the full article here.


