
Al-Ola Abdallah Shared 2025 MIDAS Trial on MRD-Guided Myeloma Therapy Published in NEJM
Al-Ola A Abdallah, Associate Professor and Plasma Cell Disorder Program Director of the Division of HMCT at the University of Kansas Medical Center, shared a post on X:
“MIDAS Trial -(Minimal Residual Disease Adaptive Strategy) trial from NEJM.
Phase 3 randomized study evaluating an MRD-adapted consolidation strategy in patients with newly diagnosed MM who are eligible for ASCT.
Important Question of the trial:
What is the benefit of ASCT in patients who achieve MRD-negative status after induction therapy?
International guidelines often recommend tandem ASCT (two ASCTs within a short period) for patients with high-risk disease!
The MIDAS trial was designed to prospectively evaluate an MRD-driven consolidation strategy to address these questions.
Study Design:
Phase 3, open-label, randomized trial conducted in 72 centers across France and Belgium.
Participants:
Transplantation-eligible pts (65 years of age or younger) with newly diagnosed MM who had completed 6 cycles of induction therapy with IsaKRd!
Patients were randomly assigned to consolidation therapy based on their MRD status after induction, as assessed using NGS at a sensitivity of 10^-5
– MRD-negative at 10^-5 sensitivity (n=485):
– ASCT group: followed by two cycles of Isa-KRd
– Isa-KRd group: 6 additional cycles of Isa-KRd
– MRD-positive at 10^-5 sensitivity (n=233)
– Tandem ASCT group: Two ASCTs within a short period
– Single ASCT group: One ASCT followed by two cycles of Isa-KRd
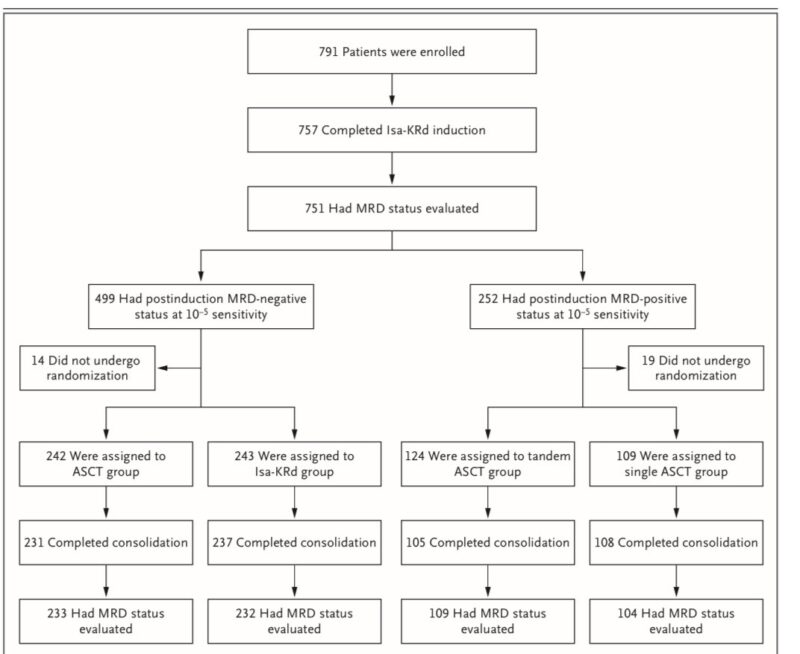
Results
Median follow-up was 16.8 months in the ASCT & Isa-KRd groups & 16.3 months in the tandem ASCT & single ASCT groups (TOO SHORT). The trial is ongoing for assessment of progression-free survival and overall survival
For patients MRD– at 10^-5 after induction (n=485):
– ASCT group: 86% achieved MRD- at 10^-6 sensitivity before maintenance therapy
– Isa-KRd group: 84% achieved MRD-status at 10^-6 sensitivity before maintenance therapy
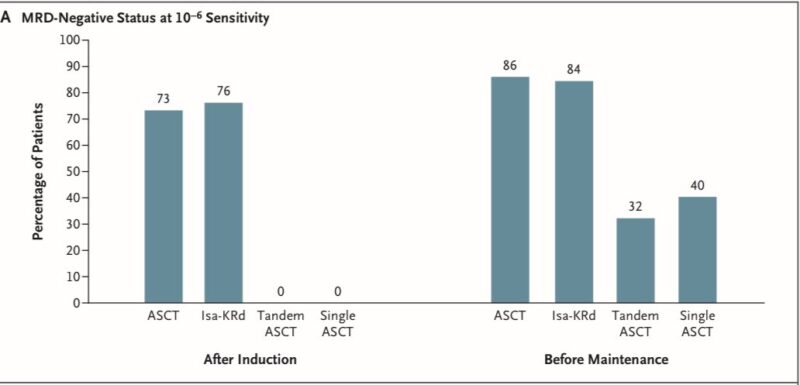
The % of patients achieving the primary end point was not significantly higher with ASCT than with Isa-KRd.
This suggests that ASCT may not offer additional benefit in this highly responsive patient subset after potent quadruplet induction.
For patients MRD + at 10^-5 sensitivity after induction (n=233):
– Tandem ASCT group: 32% achieved MRD- status at 10^-6 sensitivity before maintenance therapy
– Single ASCT group: 40% achieved MRD- status at 10^-6 sensitivity before maintenance therapy
Conclusion: The % of patients achieving the primary end point was not significantly higher with tandem ASCT than with single ASCT.
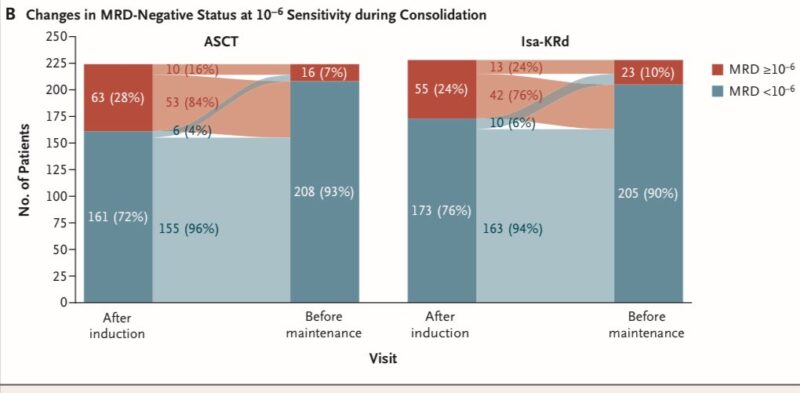
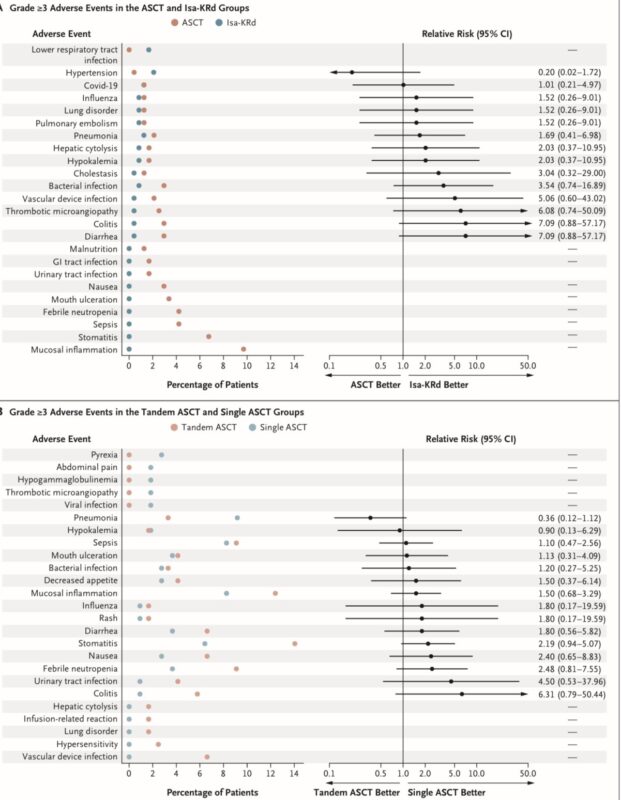
Safety:
– No new safety signals were observed during the consolidation phase compared to induction
– The incidence of individual adverse events was generally less than 10% across all groups, with exceptions being mucositis (12%) & stomatitis (14%) in the tandem ASCT group
– Disease progression occurred in 5 patients (2 in Isa-KRd, 3 in tandem ASCT groups). Two deaths unrelated to disease progression occurred in the Isa-KRd group during consolidation
Special Finding (Intresting Pattern of response!)
– Patients with the t(11;14) showed a lower rate of MRD- status at 10^-6 sensitivity after induction (24% vs. 59% without the translocation).
However, they demonstrated a delayed yet meaningful response by the start of maintenance therapy (63% vs. 78% without the translocation)!
Same question! Should we forgo ASCT in some patients in the era of quad induction therapy based on MRD status?
– The study suggests that routine tandem ASCT may not be necessary! (US doesn’t do tandem transplant) following effective induction regimens that include anti-CD38 antibodies and PI
– The trial supports the need for response-adapted therapeutic strategies that integrate both genomic and MRD-based risk stratification for optimizing myeloma treatment
– Longer follow-up is necessary to assess PFS and OS (TOO early to change our SOC = No Change in SOC for now).”
Title: Measurable Residual Disease–Guided Therapy in Newly Diagnosed Myeloma
Journal: NEJM
Authors: Aurore Perrot, Jérôme Lambert, Cyrille Hulin, Andrea Pieragostini, Lionel Karlin, Bertrand Arnulf, Philippe Rey, Laurent Garderet, Margaret Macro, Martine Escoffre-Barbe, Julie Gay, Thomas Chalopin, Romain Gounot, Jean-Marc Schiano, Mohamad Mohty, Xavier Leleu, Salomon Manier, Clara Mariette, Carine Chaleteix, Thorsten Braun, Bernard De Prijck, Hervé Avet-Loiseau, Jean-Yves Mary, Jill Corre, Philippe Moreau, Cyrille Touzeau

Jason A. Mouabbi, Assistant Professor at MD Anderson Cancer Center, shared a post on X by Al-Ola A Abdallah, adding:
“We need more trials like this for ILC.
That’s why we launched the PLUMB registry – to define MRD-adapted endpoints for drug efficacy in this often “invisible” disease.
Let’s make lobular breast cancer seen.”
More posts featuring Al-Ola Abdallah on OncoDaily.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
