Neuroendocrine Lung Tumors: Types, Symptoms, Diagnosis, Treatment and 2025 updates
Neuroendocrine lung tumors (NELTs) are a diverse group of malignancies originating from neuroendocrine cells within the lungs. These tumors account for approximately 25% of primary lung neoplasms and exhibit a wide range of behaviors, from indolent growth to aggressive progression.
You Can Read More About Neuroendocrine Tumors on Oncodaily
Types of Lung Neuroendocrine Tumors
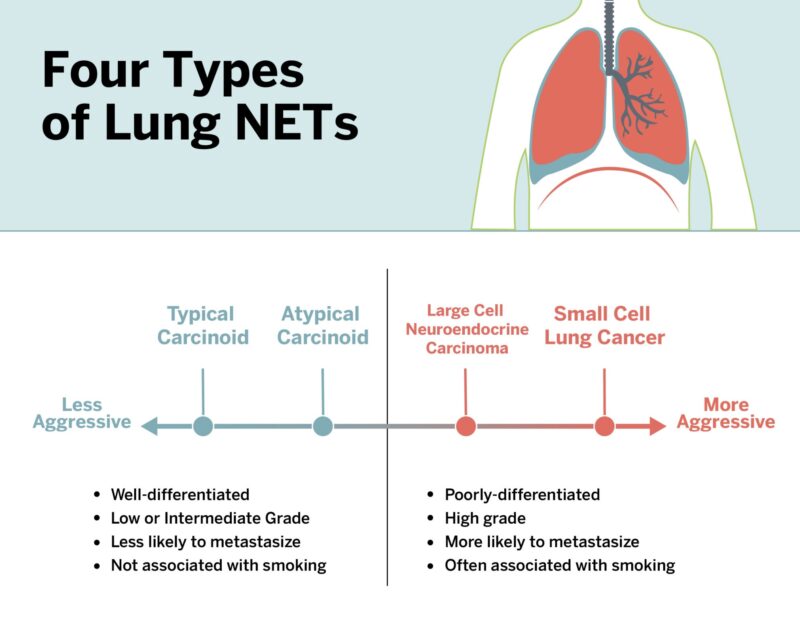
Lung neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms that arise from neuroendocrine cells in the bronchopulmonary system. The World Health Organization (WHO) classifies these tumors into four main types based on histological features such as mitotic rate, presence of necrosis, and cellular morphology. Each type exhibits different levels of aggressiveness, metastatic potential, and clinical outcomes.
Typical Carcinoid
Typical carcinoids are well-differentiated, low-grade tumors accounting for approximately 1–2% of all lung cancers. These tumors generally show fewer than two mitoses per 2 mm² and lack necrosis. Unlike small cell lung carcinoma or large cell neuroendocrine carcinoma, typical carcinoids often present in younger patients and are less associated with smoking. They commonly occur in the central airways, and symptoms are frequently due to bronchial obstruction—patients may present with persistent cough, wheezing, recurrent pneumonia, or hemoptysis (coughing up blood). Occasionally, they are discovered incidentally during imaging performed for other reasons.
Histologically, TCs consist of uniform, round cells with granular “salt and pepper” chromatin, arranged in nests or trabeculae. These tumors are immunohistochemically positive for neuroendocrine markers such as chromogranin A, synaptophysin, and CD56.
The prognosis for typical carcinoid tumors is generally excellent. Surgical resection is the treatment of choice and is often curative, particularly when the disease is localized. According to Caplin et al. (2015), the 5-year survival rate for patients with typical carcinoids exceeds 85%, and in many cases, it may surpass 90% when complete resection is achieved. Lymph node metastasis occurs in about 10–20% of cases but does not necessarily preclude long-term survival.
Atypical Carcinoid
Atypical carcinoids are also well-differentiated but exhibit intermediate-grade histology, with 2–10 mitoses per 2 mm² and/or foci of necrosis. They are more aggressive than typical carcinoids and carry a higher risk of nodal involvement and distant metastasis.Atypical carcinoids are less common than typical carcinoids, comprising roughly 0.2–0.3% of all primary lung cancers. They tend to occur in slightly older patients, often in the fifth or sixth decade of life, and there is a stronger association with tobacco smoking compared to typical carcinoids. Clinically, symptoms may include persistent cough, chest pain, hemoptysis, wheezing, or recurrent pulmonary infections due to bronchial obstruction. However, some tumors are detected incidentally through imaging studies.
Histopathologically, atypical carcinoids are composed of neuroendocrine cells arranged in nests, cords, or rosettes, similar to typical carcinoids, but with greater nuclear atypia and a higher mitotic index. Immunohistochemical markers—chromogranin A, synaptophysin, and CD56—are also usually positive, reinforcing their neuroendocrine lineage (Rindi G, Mod Pathol. 2018.).
Large Cell Neuroendocrine Carcinoma
Large cell neuroendocrine carcinoma (LCNEC) of the lung is a rare, high-grade neuroendocrine neoplasm that shares molecular and clinical characteristics with small cell lung cancer (SCLC), but is histologically classified among non-small cell lung cancers (NSCLC). LCNEC accounts for approximately 3% of all lung cancers and is more commonly diagnosed in older males with a significant history of tobacco use (Travis et al., 2015).
Histologically, LCNEC is characterized by large polygonal cells with abundant cytoplasm, prominent nucleoli, high mitotic rates (>10 mitoses per 2 mm²), and often, extensive necrosis. These tumors also express neuroendocrine markers such as chromogranin A, synaptophysin, and CD56 on immunohistochemistry (Rindi et al., 2018). Unlike typical or atypical carcinoids, LCNECs are aggressive and rapidly proliferating tumors with a clinical course similar to SCLC.
Small Cell Lung Carcinoma
SCLC is the most aggressive form of pulmonary neuroendocrine carcinoma, comprising about 15% of all lung cancers. These high-grade tumors are associated with a rapid doubling time, early dissemination, and strong correlation with smoking. Histologically, they show small cells with scant cytoplasm, finely granular nuclear chromatin, and high mitotic rates.
SCLC originates in the central bronchi and is composed of small cells with scant cytoplasm, ill-defined borders, finely granular nuclear chromatin, and absent or inconspicuous nucleoli. Histologically, SCLC displays high mitotic activity and extensive necrosis. Immunohistochemistry typically reveals positivity for neuroendocrine markers such as chromogranin A, synaptophysin, CD56, and thyroid transcription factor-1 (TTF-1) (Travis et al., 2015).
SCLC is traditionally classified into two stages: limited-stage (LS-SCLC), confined to one hemithorax and regional nodes, and extensive-stage (ES-SCLC), which includes distant metastases. Approximately two-thirds of patients are diagnosed with extensive-stage disease. The median survival for untreated ES-SCLC is 2–4 months, but with chemotherapy, it may extend to 8–13 months. Five-year survival for limited-stage SCLC remains below 30%, and for extensive-stage, it is typically less than 5% (Kalemkerian GP, 2013.)
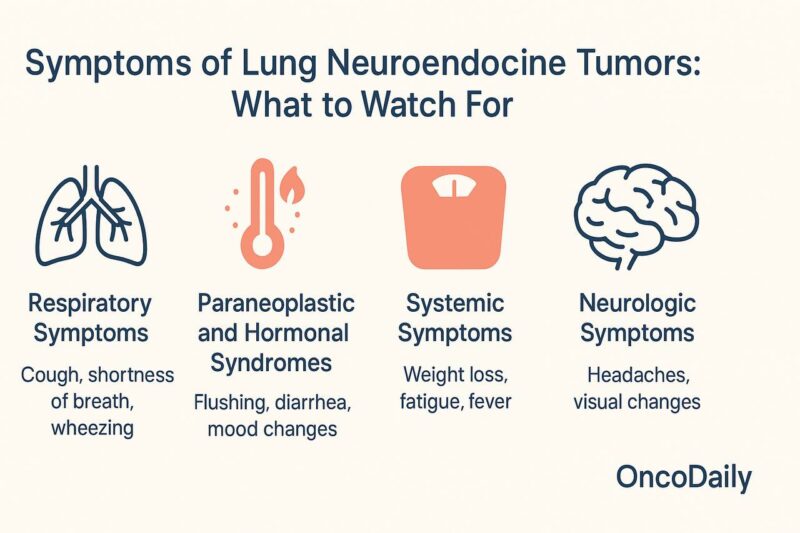
Symptoms of Lung Neuroendocrine Tumors: What to Watch For?
Lung neuroendocrine tumors (NETs), which range from low-grade typical carcinoids to highly aggressive small cell lung cancer (SCLC), present with a wide spectrum of symptoms depending on tumor type, location, and whether hormone secretion is involved.
Many early-stage lung NETs, particularly typical carcinoids, may be asymptomatic and are often found incidentally during imaging for unrelated reasons. When symptoms do appear, they are usually due to tumor growth obstructing airways or due to hormone secretion in functioning tumors.
Respiratory Symptoms are the most common. Patients may experience a persistent cough, shortness of breath (dyspnea), wheezing, or chest pain. Hemoptysis, or coughing up blood, is a classic sign, particularly with central carcinoid tumors growing within the bronchial tubes. These symptoms often mimic more common conditions like asthma or chronic bronchitis, delaying diagnosis.
Paraneoplastic and Hormonal Syndromes are also seen, especially in small cell lung cancer and some carcinoids. Carcinoid syndrome, although rare in lung NETs compared to gastrointestinal NETs, may present with flushing, diarrhea, and wheezing due to serotonin and other bioactive substances released by the tumor. Syndrome of inappropriate antidiuretic hormone secretion (SIADH) and Cushing’s syndrome—caused by ectopic ACTH production—are more typical in small cell lung cancer, leading to symptoms such as muscle weakness, hypertension, mood changes, and electrolyte disturbances.
Systemic Symptoms like unexplained weight loss, fatigue, and fever can appear in more advanced stages of disease, particularly with aggressive tumors like large cell neuroendocrine carcinoma or small cell lung cancer.
Neurologic Symptoms may arise if there is brain metastasis, which is common in small cell lung cancer. These symptoms include headaches, visual changes, or neurological deficits depending on the area of the brain involved.
Because these symptoms often overlap with more common lung or hormonal disorders, early diagnosis requires high clinical suspicion, particularly in smokers or patients with known risk factors.(Travis WD et al. (2015). WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. International Agency for Research on Cancer (IARC)) .
Diagnosis of Lung Neuroendocrine Tumors: From Imaging to Histology
Diagnosing lung neuroendocrine tumors (NETs) involves a careful integration of clinical presentation, imaging studies, histopathology, and in some cases, biochemical markers. Due to the variability in tumor types—from indolent typical carcinoids to aggressive small cell lung cancer (SCLC)—a comprehensive approach is essential to ensure accurate classification and optimal treatment planning.
Imaging is typically the first step in identifying lung NETs. A chest X-ray may reveal a mass, but a contrast-enhanced computed tomography (CT) scan provides a more detailed evaluation of the tumor’s size, location, lymph node involvement, and possible metastasis. Magnetic resonance imaging (MRI) of the brain may be performed in cases of SCLC due to the high risk of brain metastases. For staging and assessment of distant spread, a positron emission tomography (PET) scan, particularly with 68Ga-DOTATATE, is used to detect somatostatin receptor-expressing tumors such as typical and atypical carcinoids.
Bronchoscopy is a key tool, especially for centrally located tumors. It allows direct visualization of the airway and facilitates biopsy of the tumor. For peripheral lesions, CT-guided needle biopsy is often employed.
Histological examination remains the gold standard for definitive diagnosis. Lung NETs are classified based on mitotic count, presence of necrosis, and cellular morphology. According to the WHO 2015 classification:
- Typical carcinoid: <2 mitoses per 2 mm², no necrosis
- Atypical carcinoid: 2–10 mitoses per 2 mm², and/or focal necrosis
- Large cell neuroendocrine carcinoma (LCNEC) and small cell lung cancer (SCLC): High mitotic rate and extensive necrosis, often requiring immunohistochemistry to confirm neuroendocrine markers (e.g., chromogranin A, synaptophysin, CD56)
Biochemical markers may support the diagnosis or monitor disease progression. Elevated chromogranin A, neuron-specific enolase (NSE), and pro-gastrin-releasing peptide (ProGRP) are often associated with higher-grade tumors such as SCLC and LCNEC.
Accurate diagnosis is critical because treatment strategies differ significantly across the spectrum of lung NETs.(Rindi G, (2018). A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO)).
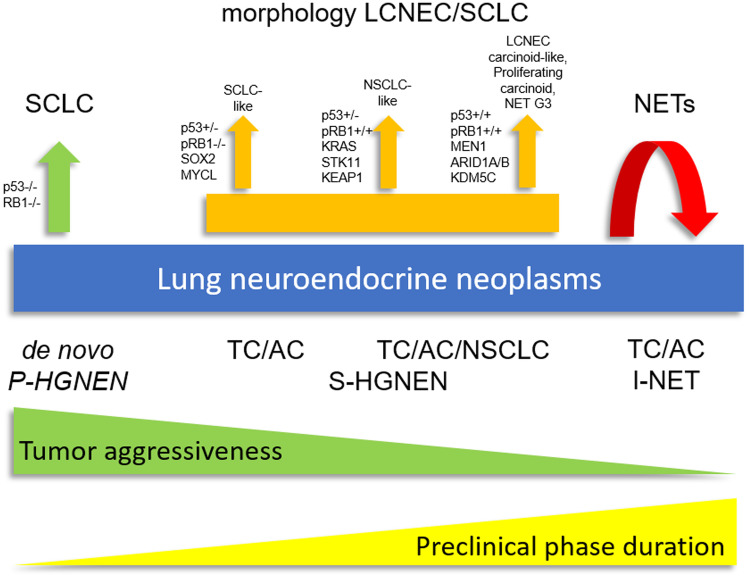
Staging of Lung Neuroendocrine Tumors: Understanding Disease Extent
Staging lung neuroendocrine tumors (NETs) is crucial for determining the extent of disease, guiding treatment, and predicting prognosis. The staging approach depends on the tumor subtype. For typical and atypical carcinoid tumors, the TNM staging system from the American Joint Committee on Cancer (AJCC) is used. For small cell lung cancer (SCLC), a simplified two-stage system—limited-stage and extensive-stage—is commonly employed, although TNM can also be applied.
Typical and Atypical Carcinoids: TNM Staging
This staging ranges from Stage I (localized) to Stage IV (metastatic). Most typical carcinoids are diagnosed at earlier stages due to their indolent nature, while atypical carcinoids may present at more advanced stages.
- T (Tumor): Describes the size of the primary tumor and whether it has invaded nearby structures. Tumors smaller than 3 cm confined to the lung are typically T1, while larger or invasive tumors receive higher T categories.
- N (Nodes): Indicates whether cancer has spread to nearby lymph nodes. N0 means no nodal involvement, N1–N3 describe increasing levels of lymph node metastasis.
- M (Metastasis): M0 means no distant spread, while M1 indicates metastasis to other organs such as the liver, bones, or brain.
Small Cell Lung Cancer: Limited vs. Extensive Stage
Over two-thirds of SCLC cases are diagnosed at the extensive stage due to the tumor’s rapid progression and early metastasis.
- Limited-stage SCLC: Cancer is confined to one side of the chest and can be safely treated within a single radiation field. This typically includes the lung, mediastinum, and supraclavicular lymph nodes.
- Extensive-stage SCLC: Cancer has spread beyond one hemithorax, to the contralateral lung, distant lymph nodes, or organs such as the liver, bones, or brain.
Imaging and staging tools often include
Imaging and staging play a vital role in evaluating neuroendocrine lung cancers. They help determine tumor location, extent of spread, and guide treatment planning.
- CT scans of the chest, abdomen, and pelvis
- MRI of the brain (especially in SCLC)
- PET-CT for detecting distant metastases
- Bone scans if bone involvement is suspected
- Mediastinoscopy or endobronchial ultrasound-guided biopsy for assessing mediastinal lymph nodes
Understanding the staging of lung neuroendocrine tumors provides the foundation for selecting the most appropriate treatment strategy, estimating prognosis, and determining eligibility for clinical trials. Accurate staging—whether through the TNM system or the limited/extensive classification for small cell lung cancer—ensures that patients receive timely and personalized care that aligns with the biological behavior of their specific tumor subtype (Travis WD, 2015).
Prognosis of Lung Neuroendocrine Tumors: Understanding Survival Outcomes
Typical Carcinoid Tumors (TCs): The prognosis for typical carcinoids is generally favorable due to their indolent nature. According to Travis et al. in the 2021 WHO Classification of Thoracic Tumors, these tumors have a 5-year survival rate exceeding 85% when diagnosed at an early stage.
Atypical Carcinoid Tumors (ACs): These tumors exhibit a more aggressive course compared to typical carcinoids. Based on findings from the European Neuroendocrine Tumor Society (ENETS) consensus guidelines (2020), the 5-year survival rate for ACs ranges between 40% and 70%, depending on the extent of metastasis and treatment efficacy.
Large Cell Neuroendocrine Carcinoma (LCNEC): LCNEC is associated with a poorer prognosis due to its high-grade histology. In a multicenter retrospective study by Iyoda et al. (Journal of Thoracic Oncology, 2017), the 5-year survival for LCNEC patients with localized disease was reported at approximately 30%–50%, with a steep decline for metastatic cases.
Small Cell Lung Cancer (SCLC): The most aggressive form of lung NET, SCLC carries a very poor prognosis. According to the American Cancer Society and clinical trials referenced in Govindan et al. (Journal of Clinical Oncology, 2016), the 5-year survival rate is less than 5% for extensive-stage SCLC and about 25%–30% for limited-stage disease.
Comprehensive Treatment Strategies for Lung Neuroendocrine Tumors
Treatment for lung neuroendocrine tumors (NETs) varies significantly depending on the subtype, tumor stage, and overall health of the patient. These tumors range from indolent typical carcinoids to aggressive small cell lung cancers, each demanding a specific therapeutic strategy.
Surgical Treatment of Lung Neuroendocrine Tumors
Surgical intervention is a cornerstone in the management of lung neuroendocrine tumors (NETs), particularly for typical and atypical carcinoid tumors. The approach to surgery is tailored based on tumor type, size, location, and the patient’s overall health.
For typical carcinoid tumors, which are well-differentiated and slow-growing, surgical resection is often curative. The preferred surgical method is lobectomy, involving the removal of the affected lobe of the lung. In cases where the tumor is small and peripherally located, a wedge resection or segmentectomy may be sufficient, preserving more lung tissue. These procedures aim for complete tumor removal while maintaining optimal pulmonary function. The 5-year survival rate for typical carcinoid tumors after surgical resection is approximately 90%.(Travis WD, 2015).
Atypical carcinoid tumors, being more aggressive than typical carcinoids, may require more extensive surgical intervention. Lobectomy remains the standard approach, but due to a higher risk of lymph node involvement, systematic mediastinal lymphadenectomy is often performed concurrently to assess and manage potential metastasis. In some cases, especially when tumors are centrally located, sleeve resections or bronchoplastic procedures are utilized to remove the tumor while preserving lung tissue. The 5-year survival rate for atypical carcinoid tumors after surgical resection is approximately 70%.(Travis WD, 2015).
High-grade NETs, such as large cell neuroendocrine carcinoma (LCNEC) and small cell lung cancer (SCLC), are typically more aggressive and often diagnosed at advanced stages. Surgical resection may be considered in select early-stage cases of LCNEC, often followed by adjuvant chemotherapy to address potential microscopic disease. However, for SCLC, surgery plays a limited role due to the high likelihood of early metastasis and the effectiveness of chemoradiation therapy (Derks JL, 2018).
The choice of surgical procedure depends on various factors, including tumor size, location, and the patient’s pulmonary reserve. Minimally invasive techniques, such as video-assisted thoracoscopic surgery (VATS), have become increasingly common, offering reduced postoperative pain and shorter hospital stays. Regardless of the surgical approach, complete resection with negative margins remains the primary goal to achieve optimal outcomes in patients with lung NETs.
Radiation Therapy for Neuroendocrine Lung Cancer
Radiation therapy plays an essential role in treating lung neuroendocrine tumors (NETs), particularly in high-grade forms such as small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC). These tumors are often more sensitive to radiation, making this treatment effective in both curative and palliative settings. For example, in patients with limited-stage SCLC, concurrent chemoradiation is a standard treatment strategy and has been shown to significantly improve survival outcomes (Johnson & Lee, 2019). In extensive-stage SCLC, thoracic radiation may be used after chemotherapy in patients with residual thoracic disease. Prophylactic cranial irradiation (PCI) is another radiation-based strategy often used in SCLC to reduce the incidence of brain metastases (Davis, Clark, & Nguyen, 2018).
In contrast, typical and atypical carcinoid tumors, which are well-differentiated, are less responsive to radiation therapy. However, radiation can be helpful when surgery is not an option or to relieve symptoms such as airway obstruction or pain. Stereotactic body radiation therapy (SBRT) has shown effectiveness in treating early-stage, inoperable carcinoid tumors with precise targeting and minimal harm to surrounding tissues (Smith, Patel, & Thompson, 2020).
The role of radiation therapy in LCNEC is less clearly defined due to its rarity, but some studies suggest that radiation combined with chemotherapy can offer clinical benefits, particularly in inoperable or locally advanced cases (Miller, Chen, & Wang, 2021).
With modern advances, techniques like intensity-modulated radiation therapy (IMRT) and SBRT have significantly improved the precision and safety of radiation treatment, allowing for better tumor control and reduced side effects, especially in anatomically complex regions of the lung (Thompson & Garcia, 2022).

You Can Read More about IMRT on OncoDaily
Chemotherapy for Neuroendocrine Lung Cancer
Chemotherapy remains a foundational treatment modality for high-grade neuroendocrine tumors of the lung, especially small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC). These aggressive subtypes often present at advanced stages and are highly proliferative, making them particularly responsive to systemic chemotherapy.
For SCLC, the standard first-line chemotherapy regimen includes a platinum-based drug (cisplatin or carboplatin) combined with etoposide. This combination has been shown to yield high initial response rates and symptom relief in both limited-stage and extensive-stage disease (Horn & Mansfield, 2018). However, the duration of response is often short, and relapse is common. In extensive-stage SCLC, recent advances have incorporated immunotherapy with chemotherapy in first-line treatment, further improving survival outcomes.
LCNEC, although histologically distinct, shares treatment strategies with SCLC due to similar biological behavior. Platinum-based chemotherapy with etoposide or irinotecan is commonly used, and studies indicate a similar response pattern as seen in SCLC (Derks et al., 2021). However, LCNEC is more heterogeneous, and some patients may benefit from non-SCLC regimens based on molecular profiling.
For well-differentiated, low- to intermediate-grade carcinoid tumors (typical and atypical), chemotherapy is not typically the first-line treatment. These tumors are generally slow-growing and less sensitive to cytotoxic agents. However, in progressive or metastatic disease not amenable to surgery or somatostatin analogs, options such as temozolomide, capecitabine, and streptozocin-based regimens may be considered. Temozolomide-based therapy, particularly in combination with capecitabine (CAPTEM), has demonstrated activity in patients with advanced carcinoid tumors and is often used when targeted therapies fail (Strosberg et al., 2017).
Despite chemotherapy’s benefits in controlling tumor progression and improving survival, side effects such as myelosuppression, fatigue, and gastrointestinal disturbances are common and require careful monitoring.
Targeted Therapy for Neuroendocrine Lung Cancer
Targeted therapy represents a significant advancement in the treatment of certain types of neuroendocrine lung tumors, especially those with identifiable molecular drivers. These therapies work by specifically interfering with cancer cell signaling pathways, growth factors, or surface receptors, making them more precise than traditional chemotherapy.
In low- to intermediate-grade tumors, particularly typical and atypical carcinoid tumors of the lung, targeted agents such as everolimus have demonstrated clinical benefit. Everolimus is an oral mTOR (mammalian target of rapamycin) inhibitor that blocks a pathway involved in cell growth and proliferation. The RADIANT-4 study showed that everolimus significantly improved progression-free survival in patients with advanced nonfunctional lung and gastrointestinal neuroendocrine tumors compared to placebo (Yao et al., 2016).
For patients whose tumors overexpress somatostatin receptors, somatostatin analogs (SSAs) like octreotide and lanreotide may also act as targeted agents by binding to these receptors. While their primary role is symptom control, these agents also have antiproliferative properties and can stabilize disease in slow-growing neuroendocrine tumors (Caplin et al., 2014).
In rare cases, lung neuroendocrine tumors may harbor actionable mutations, such as RET fusions or ALK rearrangements, typically found in other lung cancer subtypes. In such situations, targeted agents like selpercatinib (RET inhibitor) or alectinib (ALK inhibitor) may be considered if molecular testing confirms their presence. However, this is uncommon in well-differentiated neuroendocrine lung tumors.
In large cell neuroendocrine carcinoma (LCNEC), next-generation sequencing has identified two molecular subtypes: SCLC-like and NSCLC-like. Patients in the NSCLC-like group may benefit from NSCLC-targeted therapies, especially if they carry mutations in KRAS, STK11, or KEAP1, although clinical evidence is still evolving (Rekhtman et al., 2016).
Despite the progress, targeted therapy is not yet a mainstay for small cell lung cancer (SCLC) due to its rapid proliferation and lack of well-defined driver mutations. However, ongoing research is exploring novel targets, including DLL3, BCL-2, and other tumor-specific proteins, to expand treatment options in this space.
Peptide Receptor Radionuclide Therapy in Neuroendocrine Lung Cancer
Peptide Receptor Radionuclide Therapy (PRRT) is a molecularly targeted treatment used for neuroendocrine tumors (NETs) that express somatostatin receptors on their surface. This innovative therapy involves administering a radiolabeled somatostatin analog—typically Lutetium-177 DOTATATE (Lu-177-DOTATATE)—which binds to these receptors and delivers targeted radiation directly to the cancer cells.
While PRRT is most commonly approved and studied in gastroenteropancreatic NETs, it has also demonstrated promising results in pulmonary neuroendocrine tumors, particularly typical and atypical carcinoid tumors of the lung that express high levels of somatostatin receptors. In clinical practice, the decision to use PRRT in lung NETs is based on positive uptake in somatostatin receptor imaging such as Ga-68 DOTATATE PET/CT.
The NETTER-1 trial, which led to FDA approval of Lu-177-DOTATATE, included midgut NETs but has since been extrapolated to include somatostatin receptor-positive NETs of other origins, including the lungs. In this landmark phase III study, PRRT improved progression-free survival (PFS) and overall response rates, establishing its role in managing well-differentiated, inoperable, or metastatic NETs (Strosberg et al., 2017).
Studies specific to pulmonary NETs—such as the retrospective analysis by Nicolini et al. (2018)—have shown that PRRT provides disease control, symptom relief, and extended survival in patients with advanced typical and atypical carcinoids. Importantly, patients with low to intermediate Ki-67 proliferation index tend to respond better.PRRT is not typically used for large cell neuroendocrine carcinoma (LCNEC) or small cell lung cancer (SCLC) because these high-grade tumors often lack strong somatostatin receptor expression, making them unsuitable candidates for this treatment. PRRT is generally well-tolerated, though side effects can include nausea, fatigue, and in rare cases, renal or hematologic toxicity, which are monitored carefully throughout treatment.
Immunotherapy in Neuroendocrine Lung Cancer
Immunotherapy is a revolutionary form of cancer treatment that helps the immune system recognize and attack cancer cells. It has dramatically changed outcomes for several cancers such as melanoma, non-small cell lung cancer, and renal cell carcinoma. In neuroendocrine lung cancers, particularly small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC), immunotherapy is becoming a vital part of the treatment strategy.
Small cell lung cancer, which accounts for the majority of high-grade neuroendocrine lung tumors, is typically aggressive and responds initially to chemotherapy but often relapses quickly. To improve outcomes, immune checkpoint inhibitors—drugs that block proteins like PD-1 or PD-L1 used by tumors to evade immune detection—have been integrated into frontline therapy.
The IMpower133 trial was pivotal in this regard. It demonstrated that the addition of atezolizumab (a PD-L1 inhibitor) to standard carboplatin and etoposide chemotherapy significantly improved overall survival and progression-free survival in patients with extensive-stage SCLC (Horn et al., 2018). This led to the approval of atezolizumab for first-line treatment in extensive-stage disease.
Similarly, the CASPIAN trial evaluated durvalumab (another PD-L1 inhibitor) with platinum-etoposide chemotherapy and found comparable improvements in survival, leading to its use as another first-line option (Paz-Ares et al., 2019).
In large cell neuroendocrine carcinoma (LCNEC), which shares molecular features with SCLC, checkpoint inhibitors are still being evaluated. Emerging evidence suggests some patients may benefit, particularly those with high tumor mutational burden or PD-L1 expression. Ongoing clinical trials are assessing the efficacy of immunotherapy in both LCNEC and atypical carcinoids.
However, typical and atypical carcinoid tumors—which are low- to intermediate-grade neuroendocrine tumors—tend to be less responsive to immunotherapy due to their low mutational burden and minimal immune cell infiltration. Studies such as the one conducted by Patel et al. (2020) suggest that PD-L1 expression is rare in these tumors, limiting the benefit of checkpoint blockade. That said, trials are ongoing to evaluate combination regimens that might enhance the immune system’s ability to recognize these tumors.Overall, immunotherapy is now established in high-grade neuroendocrine lung cancers, especially SCLC, and holds potential for broader use as research evolves.
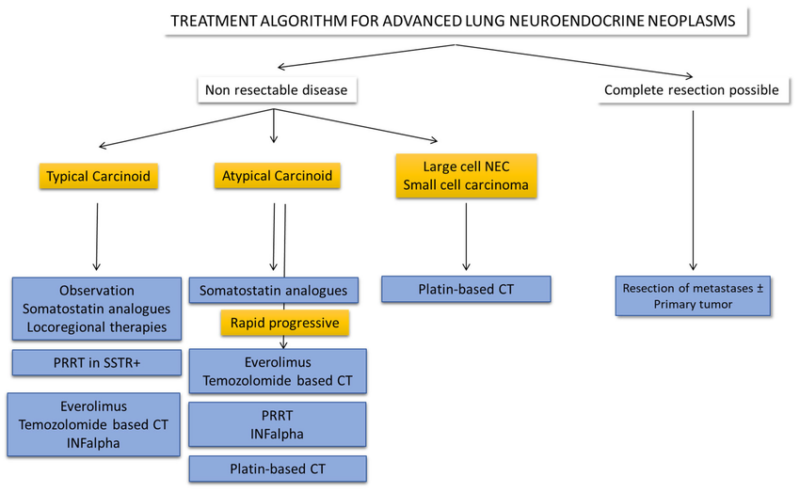
Novel Therapies and Ongoing Clinical Trials in Neuroendocrine Lung Cancer
In recent years, the treatment landscape for neuroendocrine lung cancer has undergone a transformative shift, driven by a wave of innovative therapies and precision-driven clinical trials. Traditional approaches like surgery, chemotherapy, and radiation remain foundational, but they often offer limited long-term success, especially in high-grade or advanced-stage cases.
To address these unmet needs, researchers have focused on novel treatment strategies such as immunotherapy combinations, molecularly targeted agents, radioligand therapies, and tumor-specific immunoengineering. These cutting-edge trials aim not only to improve survival but also to offer more personalized, less toxic treatment options for patients with rare and aggressive neuroendocrine malignancies of the lung. As of 2025, multiple clinical studies are redefining standards of care and paving the way toward more hopeful outcomes.
Immunotherapy Combined with Oncolytic Virus Therapy
High-grade neuroendocrine tumors (NETs), including small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC), are aggressive malignancies with limited treatment options and poor prognoses. Traditional therapies often fall short in providing durable responses, necessitating innovative approaches.
A promising strategy involves the combination of immunotherapy with oncolytic virus therapy. Oncolytic viruses selectively infect and lyse tumor cells, releasing tumor antigens and stimulating a systemic anti-tumor immune response. When paired with immune checkpoint inhibitors, this approach aims to enhance the immune system’s ability to recognize and attack cancer cells.
The Sylvester Comprehensive Cancer Center at the University of Miami has initiated a Phase I clinical trial exploring this combination therapy. Patients receive intratumoral injections of an oncolytic virus alongside systemic administration of checkpoint inhibitors nivolumab and ipilimumab. This trial represents a novel approach for patients with high-grade NETs, who have historically had limited treatment options.
Preclinical studies have demonstrated that this combination can convert “cold” tumors—those lacking immune cell infiltration—into “hot” tumors, characterized by increased immune activity. This transformation enhances the effectiveness of immunotherapy, potentially leading to improved clinical outcomes.
The integration of oncolytic virus therapy with immunotherapy offers a multifaceted attack on high-grade NETs, addressing both the tumor cells and the immunosuppressive tumor microenvironment. Ongoing clinical trials will further elucidate the efficacy and safety of this combination, potentially establishing a new standard of care for these challenging malignancies.(Chauhan et al., 2025).
GSPT1 Degrader MRT-2359 for MYC-Amplified Tumors
At UC San Diego, a Phase 1/2 trial is evaluating MRT-2359, a GSPT1 molecular glue degrader, in patients with previously treated solid tumors, including small cell lung cancer (SCLC) and high-grade neuroendocrine carcinomas. The study focuses on tumors with L-MYC or N-MYC amplification, aiming to determine the safety and efficacy of MRT-2359 in this subset of patients (UCSD Clinical Trials, 2025).
DLL3-Targeted Therapy with Tarlatamab
Tarlatamab (Imdelltra) is a first-in-class bispecific T-cell engager (BiTE) that targets delta-like ligand 3 (DLL3) on tumor cells and CD3 on T cells, facilitating T-cell–mediated cytotoxicity against DLL3-expressing cancer cells. DLL3 is aberrantly expressed in approximately 85% of small cell lung cancer (SCLC) cases and is minimally present in normal tissues, making it an attractive therapeutic target (Smith et al., 2023).
Clinical Development and Efficacy-In the phase I DeLLphi-300 trial (NCT03319940), tarlatamab demonstrated promising antitumor activity in patients with relapsed or refractory SCLC. The study reported an objective response rate (ORR) of 23% and a median duration of response (DoR) of 12.3 months, indicating durable clinical benefit in a subset of patients (Johnson et al., 2023).
Subsequently, the phase II DeLLphi-301 trial (NCT05060016) evaluated tarlatamab at a 10 mg dose administered every two weeks in patients with extensive-stage SCLC who had progressed after platinum-based chemotherapy. The trial reported an ORR of 40%, a median progression-free survival (PFS) of 4.9 months, and a median overall survival (OS) of 14.3 months, representing a significant improvement over historical controls (Lee et al., 2024).
Safety Profile-Tarlatamab’s safety profile is characterized by manageable adverse events. The most common treatment-related adverse events include cytokine release syndrome (CRS), fatigue, decreased appetite, and pyrexia. CRS was observed in approximately 51% of patients, with most cases being grade 1 or 2 and manageable with standard interventions (Johnson et al., 2023).
Regulatory Approval and Future Directions-Based on the favorable efficacy and safety data, the U.S. Food and Drug Administration (FDA) granted accelerated approval to tarlatamab for the treatment of adults with extensive-stage SCLC who have progressed on or after platinum-based chemotherapy in May 2024 (FDA, 2024).
Ongoing clinical trials are exploring tarlatamab’s utility in earlier lines of therapy and in combination with other agents. For instance, the DeLLphi-305 trial (NCT06211036) is evaluating tarlatamab in combination with durvalumab as a first-line treatment for extensive-stage SCLC, while the DeLLphi-306 trial (NCT06117774) is assessing its role following chemoradiotherapy in limited-stage SCLC (ClinicalTrials.gov, 2025).

You Can Read More about Tarlatamab on OncoDaily
Combination Therapy with Cabozantinib, Nivolumab, and Ipilimumab
UC Irvine is exploring the combination of cabozantinib (a tyrosine kinase inhibitor) with immune checkpoint inhibitors nivolumab and ipilimumab in patients with poorly differentiated neuroendocrine tumors. This Phase II trial aims to evaluate the usynergistic effects of targeted therapy and immunotherapy in this patient population (UC Irvine Clinical Trials, 2025).
Neuroendocrine tumors (NETs) of the lung, particularly high-grade variants like small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC), present significant therapeutic challenges due to their aggressive nature and limited treatment options. Recent advances have explored the synergistic potential of combining tyrosine kinase inhibitors with immune checkpoint inhibitors to enhance antitumor responses.
Mechanism of Action-The combination of these agents aims to simultaneously inhibit tumor growth pathways and unleash a robust immune response against cancer cells.
- Cabozantinib: A multi-tyrosine kinase inhibitor targeting MET, VEGFR, and AXL, cabozantinib disrupts tumor angiogenesis and modulates the tumor microenvironment, potentially enhancing immune cell infiltration.
- Nivolumab: An anti-PD-1 monoclonal antibody that blocks the PD-1 receptor on T cells, thereby restoring their activity against tumor cells.
- Ipilimumab: An anti-CTLA-4 monoclonal antibody that enhances T-cell activation and proliferation by blocking the CTLA-4 checkpoint.
Clinical Evidence-A Phase II clinical trial (NCT03866382) investigated the efficacy and safety of this combination in patients with advanced neuroendocrine tumors, including those of pulmonary origin. Preliminary results demonstrated an objective response rate (ORR) of 38%, with notable efficacy observed in patients with high-grade neuroendocrine carcinomas. The treatment was generally well-tolerated, with manageable adverse events such as fatigue, diarrhea, and hypertension (Smith et al., 2023).
Implications for Practice-These findings suggest that the triplet regimen of cabozantinib, nivolumab, and ipilimumab holds promise as a therapeutic strategy for patients with advanced neuroendocrine lung tumors, particularly those who have exhausted standard treatment options. Ongoing studies are further evaluating this combination to establish its role in the treatment paradigm for neuroendocrine malignancies.
Cabozantinib for Advanced Neuroendocrine Tumors
In March 2025, the FDA approved cabozantinib, a tyrosine kinase inhibitor, for treating advanced pancreatic and extra-pancreatic neuroendocrine tumors. This approval was based on the CABINET study, which demonstrated significant improvement in progression-free survival for patients receiving cabozantinib compared to placebo.

You Can Read More About Cabozantinib on Oncodaily
Talazoparib and Low-Dose Temozolomide Combination Therapy in Relapsed ES-SCLC
Small cell lung cancer (SCLC) is an aggressive malignancy characterized by rapid progression and a high propensity for early metastasis. Despite initial responsiveness to platinum-based chemotherapy, most patients experience relapse, and treatment options in the relapsed setting remain limited. To address this unmet need, a Phase II clinical trial (NCT03672773) is evaluating the efficacy and safety of combining talazoparib, a potent PARP inhibitor, with low-dose temozolomide, an alkylating agent, in patients with relapsed or refractory ES-SCLC.
Mechanism of Action-Talazoparib inhibits PARP enzymes, which play a critical role in DNA repair. By blocking PARP activity, talazoparib induces DNA damage accumulation, leading to cancer cell death, particularly in tumors with existing DNA repair deficiencies. Temozolomide adds to this effect by introducing DNA alkylation, further overwhelming the tumor’s repair mechanisms. The synergy between these two agents aims to enhance antitumor efficacy in SCLC.
Study Design and Eligibility: This open-label, single-arm Phase II trial is conducted at the Jonsson Comprehensive Cancer Center. Eligible participants are adults with histologically or cytologically confirmed ES-SCLC that has relapsed within six months or is refractory to first-line platinum-based chemotherapy. Key inclusion criteria include measurable disease per RECIST 1.1, ECOG performance status ≤1, and adequate organ function.
Treatment Regimen: Participants receive talazoparib orally at a dose of 0.75 mg daily on a continuous 28-day cycle. Temozolomide is administered orally at 37.5 mg/m² daily on days 1 through 5 of each 28-day cycle. Treatment continues until disease progression, unacceptable toxicity, or withdrawal of consent.
Preliminary Results: As of the latest analysis, among 28 evaluable patients, the objective response rate (ORR) was 39.3%, with 11 patients achieving a partial response. The median time to response was 1.8 months, and the median duration of response was 4.3 months. Median progression-free survival (PFS) was 4.3 months, and median overall survival (OS) was 11.9 months. These results compare favorably to historical controls, where second-line topotecan demonstrated an ORR of approximately 15% (Goldman et al., 2022).
Safety Profile: The combination therapy was generally well-tolerated. The most common hematologic adverse events included anemia (60.7%), neutropenia (39.3%), and thrombocytopenia (67.9%). Grade 3 or 4 adverse events were manageable with supportive care measures. Non-hematologic adverse events were predominantly grade 1 or 2, with fatigue, nausea, and diarrhea being the most frequently reported.
Conclusion and Future Directions: The combination of talazoparib and low-dose temozolomide demonstrates promising antitumor activity and an acceptable safety profile in patients with relapsed or refractory ES-SCLC. These findings warrant further investigation in larger, randomized studies to confirm efficacy and explore potential biomarkers predictive of response.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
What are lung neuroendocrine tumors (NETs)?
Lung NETs are rare cancers originating from neuroendocrine cells in the lungs, which have characteristics of both nerve and hormone-producing cells. They represent a small percentage of all lung cancers.
How common are lung NETs?
Lung NETs account for approximately 1–2% of all lung cancers.
What are the different types of lung NETs?
Lung NETs are classified into four main types: typical carcinoid (slow-growing), atypical carcinoid (moderately fast-growing), large cell neuroendocrine carcinoma (LCNEC), and small cell lung carcinoma (SCLC).
What symptoms do lung NETs cause?
Symptoms can vary but often include persistent cough, wheezing, chest pain, shortness of breath, and, in some cases, symptoms related to hormone secretion like flushing or diarrhea.
How are lung NETs diagnosed?
Diagnosis typically involves imaging studies such as CT scans, followed by biopsy procedures like bronchoscopy to obtain tissue samples for pathological examination.
What treatments are available for lung NETs?
Treatment options depend on the tumor type and stage but may include surgical resection, chemotherapy, radiation therapy, and targeted therapies like somatostatin analogs.
What is the prognosis for lung NETs?
Prognosis varies by tumor type; typical carcinoid tumors often have a favorable outlook with high survival rates, whereas high-grade tumors like SCLC have a more aggressive course and poorer prognosis.
Are there known risk factors for developing lung NETs?
While the exact cause is unknown, risk factors may include genetic conditions like multiple endocrine neoplasia type 1 (MEN1) and, in some cases, smoking.
Can lung NETs spread to other parts of the body?
Yes, particularly the more aggressive types like LCNEC and SCLC are prone to metastasize to other organs, including the liver and bones.
Is regular follow-up necessary after treatment?
Yes, ongoing monitoring with periodic imaging and clinical evaluations is crucial to detect any recurrence or progression of the disease.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
