“Today marks an important day for patients with bladder cancer. 82% CR rate with TAR200 monotherapy in BCG unresponsive CIS Sunrise1 FDA Priority Review. Here is the Journal of Clinical Oncology paper supporting these results”
A Novel Intravesical Drug-Releasing System Achieves 82.4% Complete Response Rate in Phase IIb SunRISe-1 Trial
On July 31, 2025, TAR-200—a first-in-class intravesical gemcitabine-releasing system—received Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non–muscle-invasive bladder cancer (NMIBC). This follows compelling efficacy and safety data from the Phase IIb SunRISe-1 trial.
What is FDA Priority Review?
Under standard review, the FDA aims to evaluate a new drug application (NDA) or biologics license application (BLA) within 10 months. However, with a Priority Review, the FDA commits to completing its review within just 6 months.
This designation is not an approval itself but indicates that the drug in question “may provide a significant advance in medical care” compared to existing therapies. It is often granted to therapies that show the potential for improved survival, better quality of life, or treatment of conditions with unmet medical needs.
Overview of BCG-Unresponsive High-Risk NMIBC
Non–muscle-invasive bladder cancer (NMIBC) accounts for nearly 75% of newly diagnosed bladder cancers. Among these, high-risk NMIBC includes carcinoma in situ (CIS), high-grade Ta, and T1 tumors. Bacillus Calmette-Guérin (BCG) immunotherapy has been the standard of care for decades. However, up to 60% of patients experience recurrence or progression, leading to the challenging category of BCG-unresponsive NMIBC.
Radical cystectomy, though potentially curative, is associated with significant morbidity and mortality and is often refused by patients. For BCG-unresponsive disease, approved alternatives include systemic pembrolizumab, intravesical nadofaragene firadenovec, and nogapendekin alfa inbakicept (NAI) + BCG. However, these therapies offer modest complete response (CR) rates, require complex administration, and may cause systemic toxicity. Until now, no bladder-sparing option offered both durability and local tolerability in this setting.
What is TAR-200 and How Does It Work?
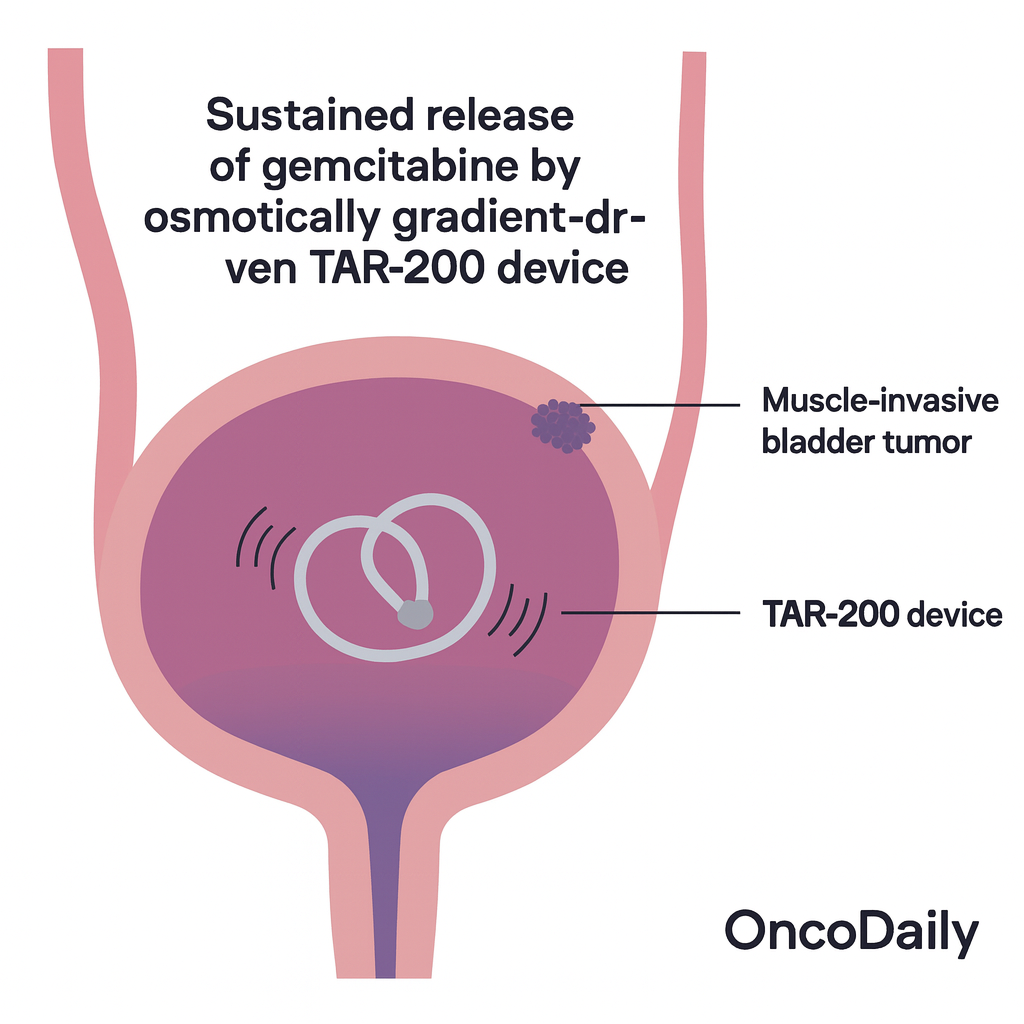
TAR-200 is a novel, passive intravesical drug delivery system designed to provide sustained release of gemcitabine directly into the bladder. Unlike conventional gemcitabine instillations with brief dwell times, TAR-200 enables prolonged local exposure without systemic absorption. This localized pharmacokinetics allows deep tissue penetration while minimizing systemic toxicity.
Each TAR-200 device is inserted in an office setting and remains in the bladder for continuous drug release over seven days. In SunRISe-1, it was administered every 3 weeks for 6 months, then every 12 weeks up to 24 months.
TAR-200 has also been studied in combination with cetrelimab, an anti–PD-1 monoclonal antibody, to explore additive immunotherapeutic effects. However, monotherapy with TAR-200 alone emerged as the optimal balance of efficacy and safety.
Study Design and Patient Population
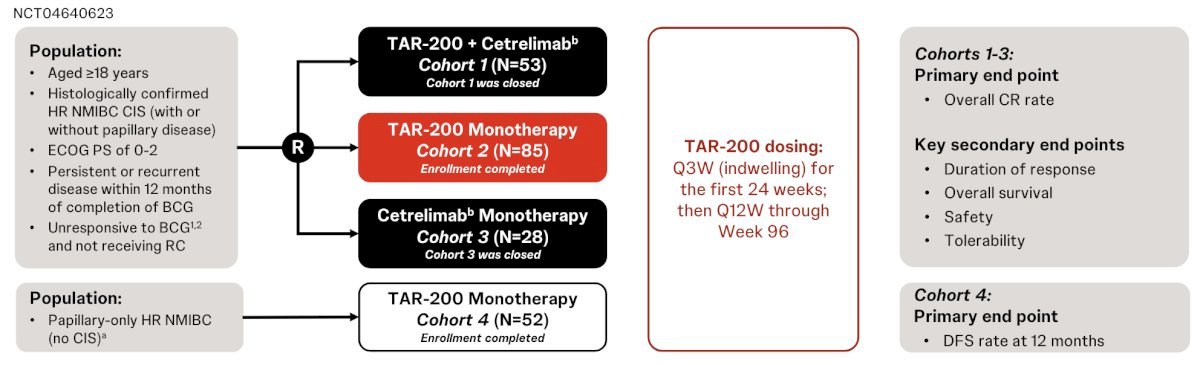
SunRISe-1 (NCT04640623) was a multicenter, open-label, Phase IIb trial evaluating TAR-200 alone or in combination with cetrelimab in BCG-unresponsive high-risk NMIBC patients ineligible for or refusing radical cystectomy.
-
Cohort 2 (C2): TAR-200 monotherapy in patients with CIS ± papillary disease
-
Cohort 4 (C4): TAR-200 monotherapy in patients with papillary disease–only NMIBC
-
Cohort 1 & 3: TAR-200 + cetrelimab and cetrelimab monotherapy, respectively
A total of 85 patients were enrolled in C2, with a median age of 71 years. Most had previously received at least 12 BCG instillations and had refused cystectomy.
Efficacy Highlights
Cohort 2 (CIS ± Papillary Disease):
-
Complete Response Rate: 82.4% (95% CI: 72.6–89.8)
-
Median Duration of Response (DoR): 25.8 months
-
12-month CR Maintenance: 45.9%
-
Radical Cystectomy-Free Rate (24 months): 75.5%
Cohort 4 (Papillary Disease Only):
-
12-month Disease-Free Survival (DFS): 70.2% (95% CI: 51.6–82.8)
These rates represent the highest to date for a bladder-sparing monotherapy in BCG-unresponsive NMIBC. Notably, responses occurred rapidly (median 2.8 months) and were consistent across age, presence of papillary disease, and PD-L1 status.
Safety Profile
TAR-200 was well tolerated with a favorable safety profile:
-
Grade ≥3 Treatment-Related AEs (C2): 12.9%
-
Most Common AEs: pollakiuria (43.5%), dysuria (40.0%), micturition urgency (24.7%), and UTI (21.2%)
-
Serious AEs: rare (5.9%), no treatment-related deaths
-
Quality of Life: Global health and physical function scores remained stable
The combination of TAR-200 and cetrelimab (C1) led to a higher rate of immune-related AEs (64.2%), while cetrelimab alone showed limited efficacy (CR: 46.4%).
How Does This Change the Treatment Landscape?
The introduction of TAR-200 marks a significant advancement in the treatment landscape of BCG-unresponsive high-risk NMIBC. Unlike traditional intravesical therapies that require prolonged bladder retention—often lasting one to two hours—TAR-200 offers a simplified and patient-friendly alternative. Its innovative design enables sustained local release of gemcitabine through a brief, office-based procedure, enhancing both convenience and tolerability while minimizing systemic exposure.
In terms of efficacy, TAR-200 delivers unprecedented results. The 82.4% complete response rate observed in patients with carcinoma in situ (CIS) and the median duration of response extending to 25.8 months establish a new therapeutic benchmark for bladder-sparing treatment. These outcomes reflect both the potency and durability of TAR-200 as a monotherapy in a population with otherwise limited options.
Perhaps most notably, the ability of TAR-200 to delay or entirely avoid radical cystectomy for the majority of patients represents a transformative shift. Given the substantial morbidity and quality-of-life impact associated with cystectomy, TAR-200 offers a viable, organ-preserving alternative that many patients find preferable.
Beyond its current indication, TAR-200 continues to be evaluated in a broader clinical development program. Ongoing studies—including SunRISe-2, SunRISe-3, and SunRISe-4—are exploring its use in earlier disease stages and in combination with systemic immunotherapies. This expanding pipeline underscores TAR-200’s potential to reshape the future of localized bladder cancer management across multiple patient populations.
What People Are Saying?
Sia Daneshmand, Professor of Urology and Medicine (Oncology)-Clinical Scholar, Director of Urologic Oncology and Clinical Research at Keck Medicine of USC — shared on LinkedIn:
“Just published online in @JCO_ASCO. SunRISe1 Phase IIb results show TAR-200 (novel intravesical drug releasing system). CR: 82% (TAR-200 alone)
Durable responses (median DOR 25.8 mo) and low toxicity. A game-changer for pts with NMIBC”
Bill Gadless, Founding Partner, emagineHealth — shared on LinkedIn:
“Johnson and Johnson’s FDA Priority Review for TAR-200: This isn’t just another bladder cancer drug. TAR-200 is a drug delivery innovation that could reset oncology expectations.It’s an intravesical delivery system that releases therapy directly into the bladder over time – ideal for high-risk, BCG-unresponsive non-muscle invasive bladder cancer, where options are limited and recurrence is brutal.
The FDA just granted Priority Review because the data demands attention:– Phase 2b SunRISe-1: 82.4% complete response rate– 52.9% remained cancer-free at least one year after response– Most adverse reactions were mild or moderateWhy this matters beyond bladder cancer:– Precision delivery is overtaking systemic therapies– Device + drug combos are redefining innovation– Pharma is going smaller, local, and more targeted
The future isn’t just better drugs. It’s better ways of delivering them.
Yüksel Ürün, Medical Oncologist, Prof. Dr. at Ankara University — shared on X:
“TAR-200 for BCG-unresponsive high-risk NMIBC.
CR: 82%, DOR: 26 months (TAR-200 alone)
Best risk-benefit: TAR-200 monotherapy—better than combo or PD-1 alone.”

Shilpa Gupta, Oncologist, Clinical Trialist, Director of GU Oncology at Cleveland Clinic — shared on X:
“Congrats Sia Daneshmand, Andrea Necchi and team on leading this game changing trial of TAR 200 in BCG unresponsive NMIBC. A big step forward towards allowing patients to avoid cystectomy.”
You can read the full article on Journal Of Clinical Oncology