The ESMO TAT Asia 2025 (European Society for Medical Oncology’s Targeted Anticancer Therapies Asia Congress) gathered oncology leaders in Singapore this July, showcasing some of the most cutting-edge breakthroughs in immunotherapy. As a hub for early-phase trials and translational oncology, TAT Asia 2025 was the place to watch for first-in-human data, biomarker strategies, and next-gen cell therapies.
Here are the top presentations and clinical updates in immunotherapy from ESMO TAT ASIA 2025 that every oncologist, researcher, and biotech leader should know about.
Cracking the Code of CAR-T Cell Response in Gastric Cancer – CT041 & Claudin18.2
One of the most anticipated talks came from Haoxin Peng (Beijing, China), exploring why some patients respond better to Claudin18.2-specific CAR-T therapy (CT041) for gastric cancer.
Key Takeaways:
- GZMK+ CD8+ T cells in a progenitor exhausted-like (Tpex) state were highly enriched in responders.
- These immune cells had predictive power for CT041 response (AUC: 0.824) and were linked to longer survival (PFS: 9.98 vs. 5.45 months).
- Tumors resistant to CT041 therapy showed immunosuppressive interactions involving IQGAP3+ cancer cells and IL6+ fibroblasts.
- Spatial transcriptomics and multiplex immunofluorescence revealed dynamic interactions between CAR-T cells and the tumor microenvironment.
Why it matters: This research could revolutionize biomarker-guided CAR-T therapy for gastric cancer by helping tailor treatment strategies based on patient-specific tumor-immune profiles.
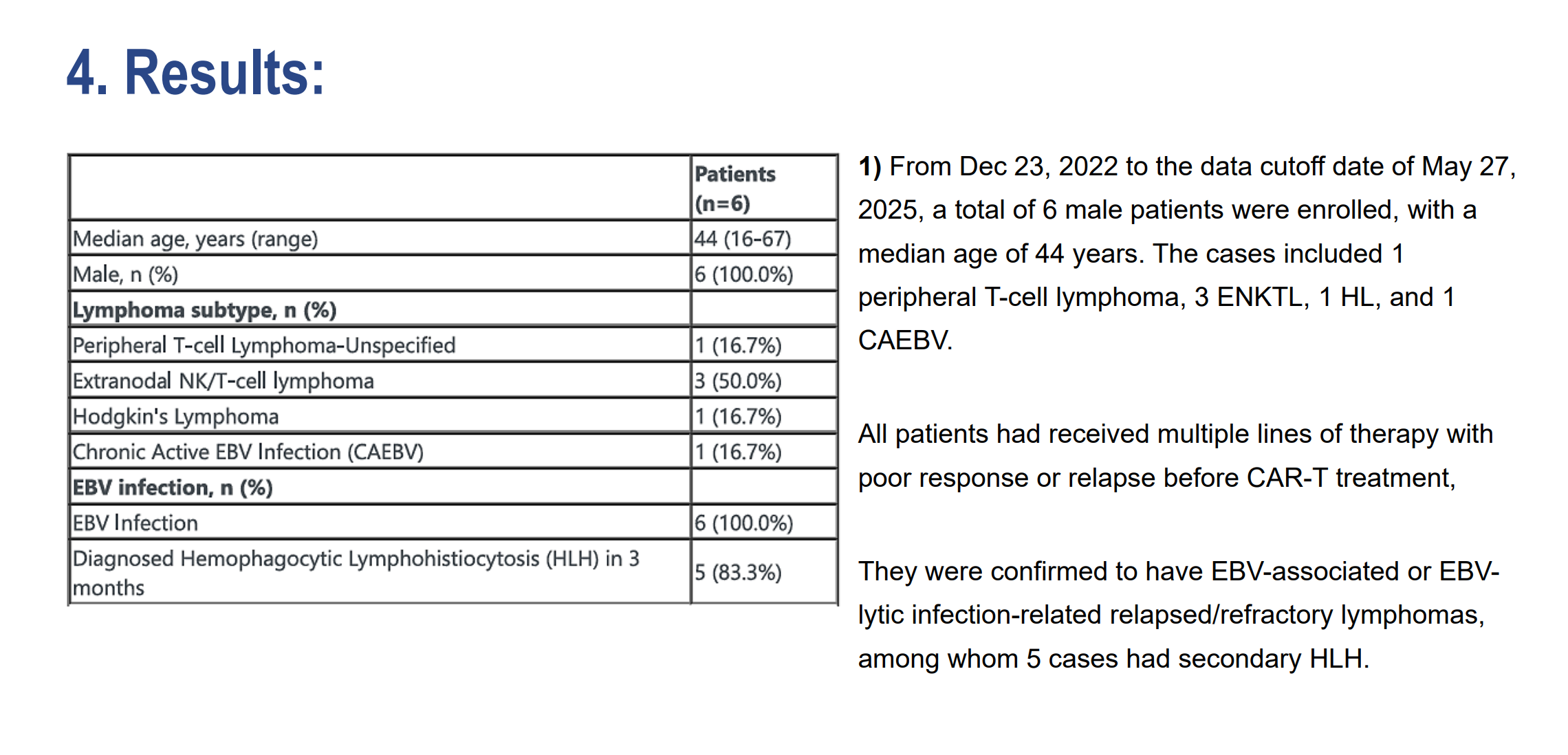
Targeting EBV+ Lymphoma with GP350 CAR-T Therapy (24O)
Fan Wu’s team from China presented exciting early-phase data on GP350-targeted CAR-T cells for relapsed/refractory EBV-associated lymphoma.
Source: ESMO TAT ASIA 2025 Meeting Resources, Author Slides
Highlights:
- 6 patients treated with anti-GP350 CAR-T cells showed high disease control rates (83.3%) and strong viral suppression.
- 50% achieved full EBV clearance, with one complete response and one partial response.
- No neurotoxicity reported; only mild CRS in most cases.
Bottom line: This study shows strong anti-viral and anti-tumor activity, positioning GP350 CAR-T as a promising approach in EBV+ lymphoma—a population with historically poor outcomes.
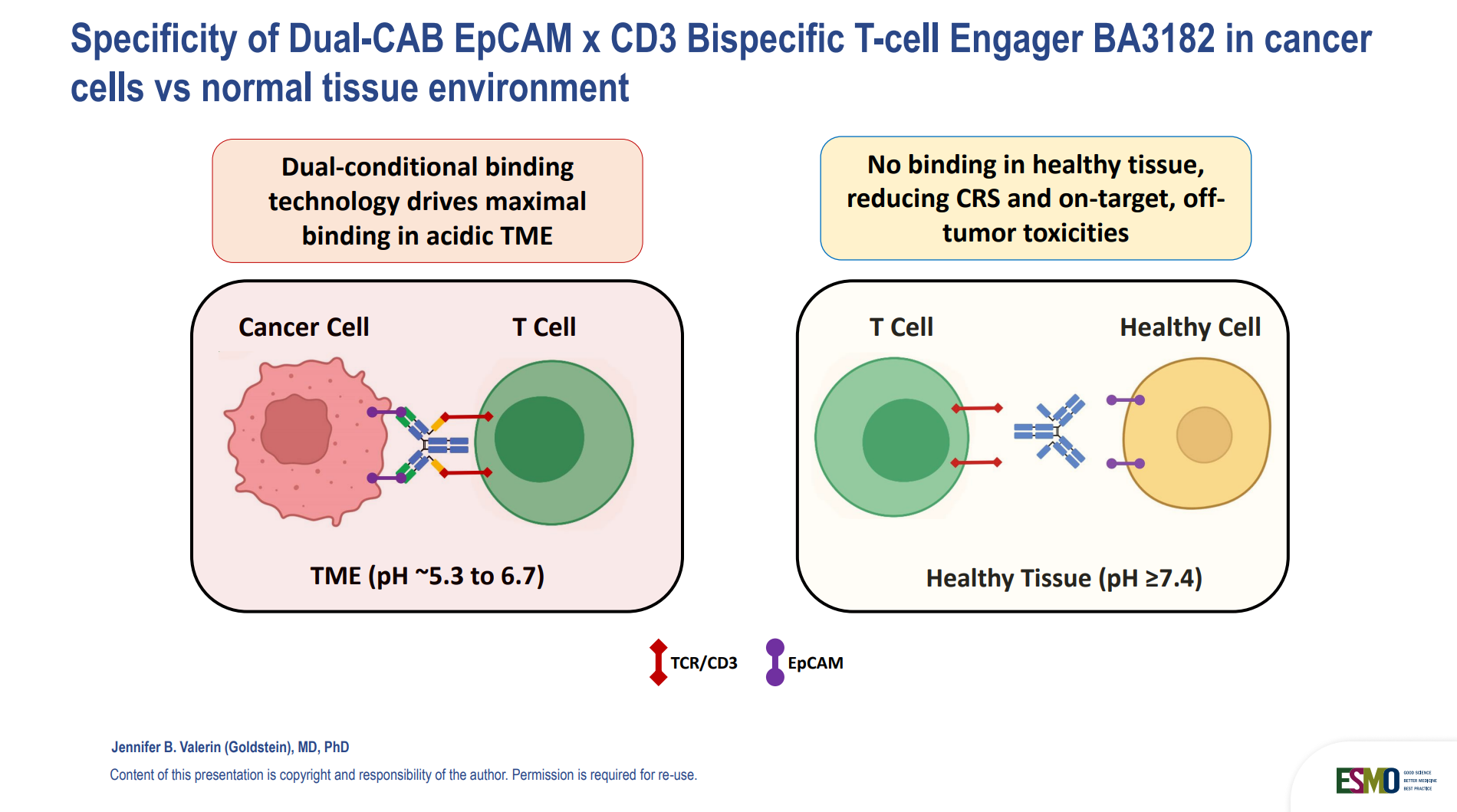
BA3182: A Next-Gen Bispecific T-Cell Engager with Tumor-Specific Activation (36O)
A novel EpCAM x CD3 bispecific engager (BA3182) was presented by Jennifer Valerin (USA) in its first-in-human trial. Designed to be conditionally active in acidic tumor environments, BA3182 aims to minimize damage to healthy tissues.
Findings:
- Early responses included tumor shrinkage in colorectal, breast, and lung cancer patients.
- Two CRC patients saw prolonged PFS (8 and 14 months).
- Manageable safety profile with mild CRS only.
Why you should care: BA3182 introduces a new class of tumor microenvironment-sensitive immunotherapies, offering precision activation with minimized toxicity.
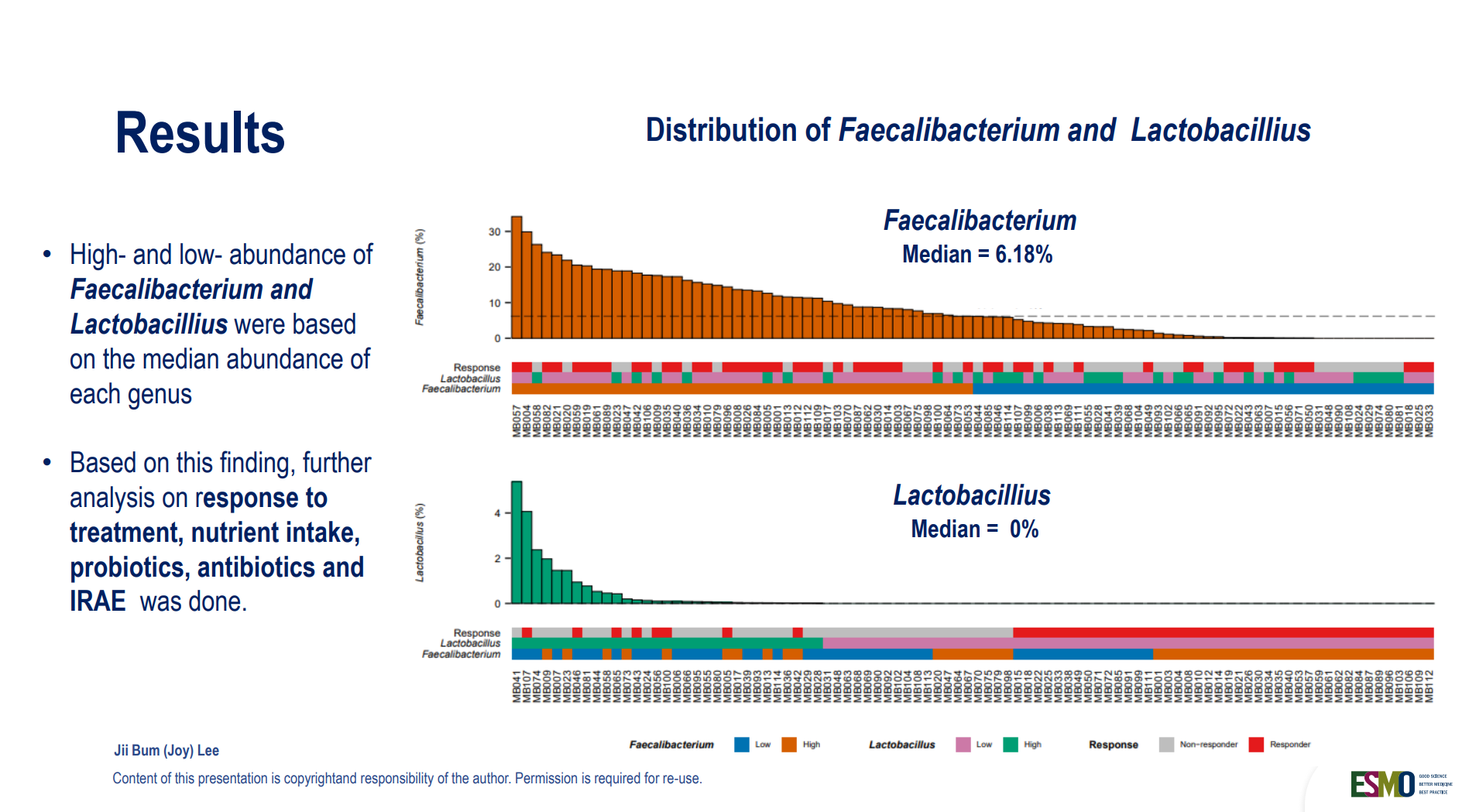
Gut Microbiome Predicts Immunotherapy Response in NSCLC (38O)
A must-watch presentation from Jii Bum Lee (Korea) highlighted how gut bacteria composition can forecast outcomes in metastatic non-small cell lung cancer (NSCLC) treated with pembrolizumab ± chemotherapy.
Author Slides
Key insights:
- Faecalibacterium-rich microbiomes were linked to longer PFS and OS.
- Lactobacillus-dominant profiles were associated with worse outcomes.
- Antibiotics lowered Faecalibacterium abundance, potentially diminishing treatment efficacy.
Take-home message: Microbiome profiling could soon become a companion diagnostic for checkpoint inhibitors, especially in first-line NSCLC.
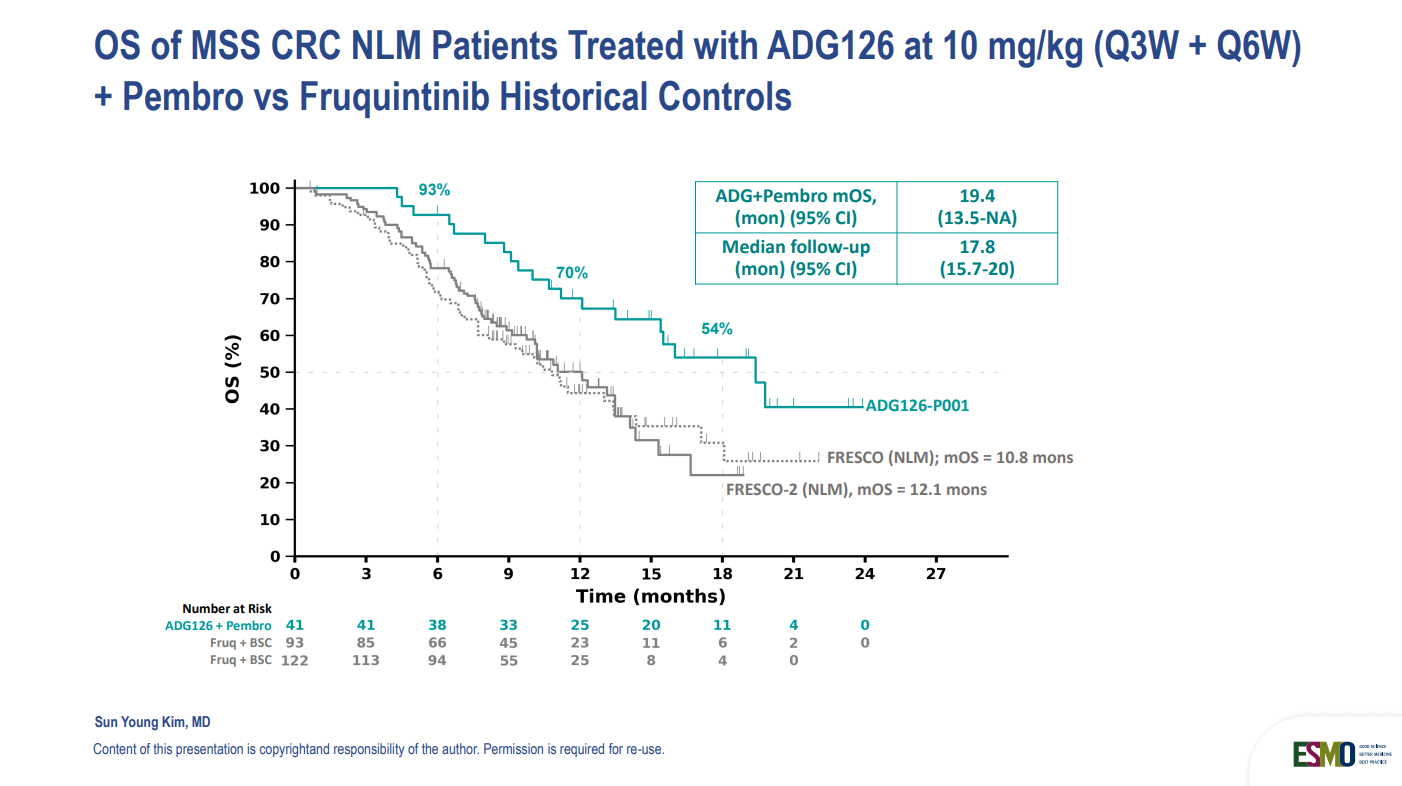
ADG126 + Pembrolizumab: Optimized Immunotherapy for MSS CRC (39O)
Microsatellite stable (MSS) colorectal cancer has long resisted immunotherapy—but ADG126, a masked anti-CTLA-4 antibody, is changing the game when combined with pembrolizumab.
Presentation by: Sun Young Kim (Korea)
What’s new:
- Promising ORR (33%) in selected MSS CRC patients (no liver mets).
- Long PFS and OS in multiple dosing cohorts.
- Low discontinuation rate and manageable toxicity, especially at lower dose intervals.
Clinical impact: This is one of the few checkpoint-based regimens showing meaningful responses in MSS CRC, marking a potential shift in the standard of care.
Social Buzz: What the Oncology World Is Saying about #TAT25
TAT Asia 2025 was trending across oncology Twitter (now X), with experts and KOLs sharing real-time impressions. Here’s a sample of what’s being said:
UCI Health Physicians shared on LinkedIn:
“At the 2025 European Society for Medical Oncology Targeted Anticancer Therapies (ESMO TAT) Asia Congress this past week, Jennifer B. Valerin, MD, PhD, associate clinical professor at the UCI Health Chao Family Comprehensive Cancer Center, presented early findings from a Phase 1 clinical trial investigating BA 3182, a conditionally active bispecific T cell engager (BiTe) therapy targeting EpCAM and activated T cells.
This clinical approach is designed to activate only in low pH tumor environments and early data shows that the drug is relatively well tolerated due to this conditional mechanism. Preliminary data also shows signs of prolonged disease stability, particularly in colorectal cancer, as this trial currently has 22 patients enrolled to date. As the principal investigator from UC Irvine, Valerin looks forward to sharing more efficacy data later this year.
Learn more about this trial and refer a patient.”
Annie Wong, Senior Lecturer at The University of Auckland, shared on X:
“Great first day ESMOTATAsia25
Great cellular therapies panel:
- on-target off-tumour tox
- when to best use it/best combo
APODDC survey highlighting barriers for trials and need for collabs.”
Later she added:
“Always so inspiring Lillian L. Siu’s Keynote insights ESMOTATAsia25
Play to our strengths and opportunities to collab/complementary trials
Strategies to improve equity/access to trials
Global visions for P1 trials to be patient focus, not just drugs focused.”
Omali Pitiyarachchi, Visiting Medical Officer (VMO) – Medical Oncologist at Prince of Wales Private Hospital, also shared a post:
“Professor Aya Helali -precautions/pitfalls in reporting exploratory biomarker analyses (No single guideline addresses this); Professor Herbert Loong increasing importance of PROs in early phase clin trials incl dose esc decisions-it’s complicated!”
Leslie Gilham, Chair, Consumer Advisory Panel at Breast Cancer Trials, shared on X:
“Honoured to be presenting in the same session as Professor Tak Mak highlighting the importance of a strong ecosystem in biotech and the importance of immunology. Thank you ESMO for including the patient voice in this very important conversation.”







