Tarlatamab is an investigational bispecific T-cell engager (BiTE®) antibody designed to target DLL3 (delta-like ligand 3) on tumor cells and CD3 on T cells. It enhances the immune system’s ability to attack DLL3-expressing tumors, extensive small cell lung cancer (SCLC), and other neuroendocrine cancers.
Which company produced Tarlatamab?
Tarlatamab, marketed under Imdelltra, was developed by Amgen Inc. It received accelerated approval from the U.S. Food and Drug Administration (FDA) on May 16, 2024, for the treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC) who have experienced disease progression on or after platinum-based chemotherapy. Regarding the patent expiration for Tarlatamab, specific details are not readily available in public sources. Generally, patents for biological drugs last for 20 years from the filing date.
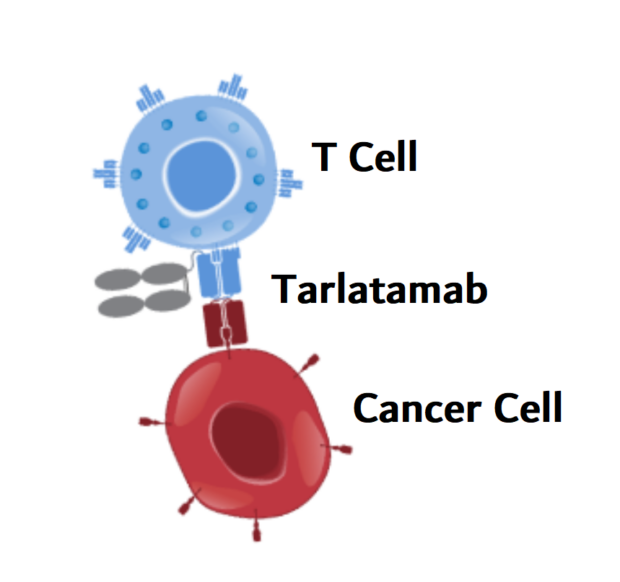
How does Tarlatamab work?
Tarlatamab, marketed as Imdelltra, is a bispecific T-cell engager (BiTE®) antibody that harnesses the body’s immune system to fight small cell lung cancer (SCLC) and other DLL3-expressing neuroendocrine tumors. Its unique mechanism of the action revolves around targeting two key molecules—DLL3, a protein found almost exclusively on cancer cells, and CD3, a receptor on T cells responsible for activating the immune response. Once administered, Tarlatamab binds to DLL3 on tumor cells, marking them as targets, while engaging CD3 on cytotoxic T cells. By bridging these two elements, Tarlatamab brings the T cells into direct contact with the cancer cells.
This proximity triggers T-cell activation, prompting them to release cytotoxic granules containing perforin and granzymes, which induce apoptosis (programmed cell death) in the tumor. As the immune response is amplified, additional T cells are recruited to continue the attack, leading to a sustained anti-tumor effect. One of the major advantages of Tarlatamab is its high specificity for DLL3, minimizing potential damage to normal tissues. Unlike CAR-T therapy, which requires engineering a patient’s T cells in a lab, Tarlatamab works without genetic modification, making it a more accessible off-the-shelf treatment.
How Is Tarlatamab Different from Traditional Immunotherapy?
Unlike conventional immunotherapies that rely on boosting the body’s general immune response, Tarlatamab actively redirects T cells to attack cancer cells in a highly targeted way. Traditional treatments, such as PD-1/PD-L1 inhibitors, work by removing immune system brakes, allowing T cells to recognize and fight tumors more effectively. However, these therapies depend on the presence of pre-activated T cells and may not work well in tumors that evade immune detection, such as small cell lung cancer (SCLC).
Tarlatamab takes a different approach by acting as a bridge between T cells and cancer cells, ensuring direct immune engagement even in tumors that typically resist traditional immunotherapy. By binding to DLL3, a protein found almost exclusively on SCLC cells, and CD3, a receptor on T cells, Tarlatamab forces the immune system to attack, even when the tumor environment is immunosuppressive. This precision-driven mechanism makes it particularly promising for cancers like SCLC, where other immunotherapies have had limited success.
What Cancers is Tarlatamab Approved to Treat?
Tarlatamab (Imdelltra) is currently FDA-approved for the treatment of extensive-stage small cell lung cancer (ES-SCLC) in adult patients who have experienced disease progression after platinum-based chemotherapy.
What research is behind the approval?
The DeLLphi-300 and DeLLphi-301 trials have been pivotal in evaluating Tarlatamab’s efficacy and safety.
The phase II DeLLphi-301 study demonstrated that Tarlatamab has durable antitumor activity in heavily pretreated extensive-stage small-cell lung cancer (ES-SCLC). The study, aligned with the FDA’s Project Optimus, compared 10 mg and 100 mg doses, selecting 10 mg for future trials due to a better benefit-risk profile. The 10 mg dose achieved a 40% objective response rate (ORR) and a median overall survival (OS) of 14.3 months, far surpassing the historical 15% ORR benchmark.
Results were presented at ESMO 2023 and published in NEJM. In the study, 220 patients, previously treated with a median of two therapies, received tarlatamab every two weeks. At 10 mg, ORR was 40% versus 32% at 100 mg. Median PFS was 4.9 months and 3.9 months, respectively, while OS at nine months was 68% and 66%. Cytokine release syndrome (CRS) was the most common adverse event, occurring in 51% (10 mg) and 61% (100 mg) of patients, primarily in cycle 1. Only 3% of patients discontinued treatment due to side effects. Given the immunosuppressive microenvironment of SCLC, tarlatamab represents a novel immunotherapeutic approach.
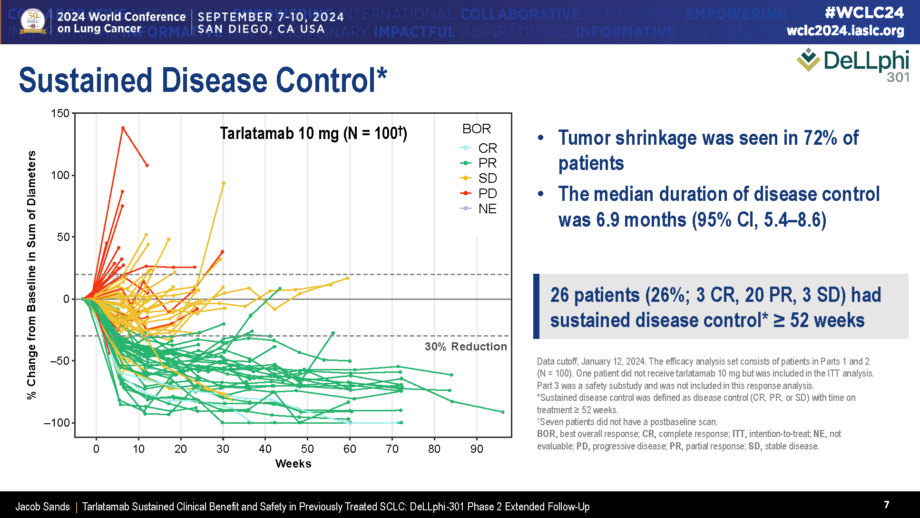
Data presented at #WCLC24 on DeLLphi-301
On December 6-8 in ESMO Asia, which was held in Singapour, The phase II DeLLphi-301 study analyzed the efficacy and safety of Tarlatamab in previously treated small-cell lung cancer (SCLC), with a subgroup analysis focused on Asian patients. Tarlatamab, a bispecific T-cell engager targeting DLL3, demonstrated promising clinical outcomes in this population. Among 41 Asian patients receiving tarlatamab 10 mg every two weeks, the objective response rate (ORR) was 46.3%, and the median duration of response (DoR) was 7.2 months. The median progression-free survival (PFS) was 5.4 months, while the median overall survival (OS) reached 19.0 months, with an 18-month OS rate of 53.3%.
DeLLphi-300 phase I study’s results, published in JCO on August 29, 2024, highlight durable responses and survival benefits, with findings previously presented at ELCC 2024. Notably, 29.4% achieved sustained disease control (≥52 weeks). Across all dose cohorts (152 patients), ORR was 25.0%, median DoR 11.2 months, and median OS 17.5 months. CNS tumor shrinkage (≥30%) occurred in 62.5% of patients with baseline brain lesions (≥10 mm), including those treated long after prior radiotherapy. No new safety concerns emerged. Given the poor outcomes of current second-line SCLC treatments (median DoR 3.3–5.3 months, OS 5.8–9.3 months), tarlatamab offers a promising.
Tarlatamab Combinations and Treatment Outcomes
Ongoing research is exploring its efficacy in combination with other therapies to enhance clinical outcomes.
Combination with Standard Chemotherapy and PD-L1 Inhibitors
The DeLLphi-303 Phase 1b trial explored the use of IMDELLTRA (tarlatamab) combined with a PD-L1 inhibitor as a first-line maintenance therapy for extensive-stage small cell lung cancer (ES-SCLC) following chemoimmunotherapy. The study demonstrated a favorable safety profile, sustained disease control, and encouraging survival outcomes, supporting further investigation in Phase 3 trials. Patients received Tarlatamab with either Durvalumab or Atezolizumab, showing similar disease control rates (62.5%) across both groups. Those treated with Durvalumab achieved a median progression-free survival (mPFS) of 5.3 months and a 9-month overall survival (OS) rate of 91.8%, while patients on Atezolizumab had an mPFS of 5.6 months and a 9-month OS rate of 86.7%.
The combination was generally well tolerated, with low rates of severe toxicity. Cytokine release syndrome (CRS) was mostly mild (grade 1-2), occurring early in treatment and manageable with supportive care. Neurotoxicity (ICANS) was rare, with lower incidence in the Durvalumab group. These findings highlight tarlatamab’s potential as a breakthrough first-line maintenance option in ES-SCLC, paving the way for further evaluation in the ongoing Phase 3 DeLLphi-305 trial.
Comparison with Standard Chemotherapy
The DeLLphi-304 Phase III trial is comparing tarlatamab to standard-of-care chemotherapy in patients with relapsed SCLC. This randomized, multicenter study aims to assess whether Tarlatamab offers superior efficacy and safety compared to traditional chemotherapy regimens in the relapsed setting.
The Side Effects of Tarlatamab and its Management
One of the most significant concerns is cytokine release syndrome (CRS), which affects more than half of the patients receiving tarlatamab. This reaction can cause fever, chills, breathing difficulties, vomiting, and extreme fatigue. CRS typically occurs during the early treatment cycles, and while most cases are mild to moderate, severe reactions require immediate medical attention. Neurological side effects are another critical risk, as Tarlatamab can cause immune effector cell-associated neurotoxicity syndrome (ICANS). Patients may experience confusion, difficulty with speech or memory, and, in severe cases, seizures.
Additionally, Tarlatamab can increase the risk of serious bleeding, making it crucial to watch for symptoms such as severe stomach pain, black or bloody stools, or coughing up blood. More commonly reported side effects include fatigue, fever, nausea, constipation, decreased appetite, and muscle or bone pain. Some patients also experience dysgeusia (altered taste), respiratory symptoms such as cough or shortness of breath, and changes in blood cell counts, including anemia and low white blood cell levels, which may increase the risk of infections. Liver and kidney function abnormalities have been observed, with some patients experiencing elevated liver enzymes and increased creatinine levels. Metabolic changes, such as low sodium, potassium, or magnesium levels, are also frequent.
While Tarlatamab presents a new and effective treatment option for ES-SCLC, careful monitoring is essential to manage potential side effects. Patients should promptly report any concerning symptoms to their healthcare provider to ensure early intervention and appropriate supportive care.
What is the Recommended Dosage of Tarlatamab?
Tarlatamab is administered as an intravenous infusion following a step-up dosing schedule to help patients tolerate treatment and minimize the risk of severe reactions. The treatment begins with Cycle 1, where patients receive an initial low dose of 1 mg on Day 1, followed by 10 mg on Days 8 and 15. In Cycle 2 and beyond, the standard dose of 10 mg is maintained, given on Days 1 and 15 of each cycle. This schedule continues through Cycles 3 and 4, and from Cycle 5 onward, patients continue to receive 10 mg every two weeks until disease progression or unacceptable toxicity occurs.
How is Tarlatamab administered?
Tarlatamab can be administered through an IV catheter that is already in use for other medications. Before infusion, the catheter should be flushed with 0.9% NaCl over 3-5 minutes to ensure patency. The medication is infused over one hour at a constant flow rate of 250 mL/hr using a programmable, lockable infusion pump equipped with an alarm for safety.
Source of Photo: Healthline
For storage, unopened vials must be kept refrigerated at 2-8ºC (36-46ºF) in their original carton, protected from light, and should not be frozen. If necessary, they can be stored at room temperature (20-25ºC or 68-77ºF) for up to 24 hours while still in their carton. Once prepared, the infusion bag must be used within 8 hours at room temperature or within 7 days if refrigerated. Preparing infusion bags should not be re-refrigerated after being removed from cold storage and must be discarded once the maximum storage time is reached.
What to Avoid During Tarlatamab Treatment?
During Tarlatamab treatment, certain precautions should be taken to ensure safety and effectiveness. Patients should avoid medications or substances that could interfere with the drug’s action or worsen side effects. It is essential to follow the healthcare provider’s instructions and avoid stopping or starting any medications without medical advice. Since Tarlatamab can cause cytokine release syndrome (CRS) and other immune-related side effects, activities that may put excessive strain on the body, such as intense physical exertion, should be approached with caution.
Additionally, alcohol and certain herbal supplements that affect the immune system should be avoided, as they may increase the risk of adverse reactions. Patients should also be mindful of potential infections, as tarlatamab affects the immune response. Avoiding crowded places, practicing good hygiene, and promptly reporting any signs of infection, such as fever or fatigue, to a healthcare provider are important. Patients with severe active infections, uncontrolled autoimmune disorders, or a history of severe allergic reactions to similar biologic therapies may not be eligible for Tarlatamab treatment. Regular monitoring and adherence to prescribed premedications can help minimize complications and improve treatment outcomes.
How effective is Tarlatamab?
While its approval is specific to SCLC, clinical trials are investigating its potential for treating other DLL3-expressing neuroendocrine tumors, such as Neuroendocrine carcinomas (NECs) of various origins and Large cell neuroendocrine carcinoma (LCNEC). In JTO, a case report for Large Cell Neuroendocrine Carcinoma in Young Adults was published. A 20-year-old man with metastatic large cell neuroendocrine carcinoma (LCNEC) of the lung, was treated with the DLL3-targeting bispecific T-cell engager tarlatamab.
Following initial responses to chemotherapy and immunotherapy, his disease progressed, and he was granted compassionate use of tarlatamab. Treatment led to a partial response lasting six months but was complicated by grade 3 cytokine release syndrome (CRS), requiring intensive care. Prophylactic tocilizumab in subsequent doses reduced CRS severity, allowing outpatient administration. His case suggests that DLL3-targeting therapies may be a promising approach for LCNEC, warranting further study. This report highlights the potential of BiTE therapies beyond SCLC and emphasizes the need to refine biomarkers for patient selection.
Ongoing trials with Tarlatamab
DeLLphi-305 is a Phase 3 clinical trial that is testing an investigational drug called Tarlatamab. This clinical trial aims to see how safe and effective Tarlatamab is at treating patients with small-cell lung cancer (SCLC). Participants will receive either Tarlatamab in combination with Durvalumab (Imfinzi®) or Durvalumab alone.
DeLLphi-306 is a Phase 3 clinical trial that is testing an investigational drug called Tarlatamab. This clinical trial aims to see how safe and effective Tarlatamab is at treating patients with small-cell lung cancer (SCLC) that have not progressed after they have completed chemoradiation. Participants will receive either Tarlatamab or a placebo.
Written by Mariam Khachatryan, MD
FAQ
What is Tarlatamab, and how does it work?
Tarlatamab is a bispecific T-cell engager (BiTE) that targets delta-like ligand 3 (DLL3), a protein commonly expressed in small-cell lung Cancer (SCLC) and other neuroendocrine tumors. It works by binding to both DLL3-expressing tumor cells and CD3-expressing T cells, activating the immune system to destroy cancer cells.
What types of cancer is Tarlatamab used to treat?
Tarlatamab is primarily being studied for extensive-stage small cell lung cancer (ES-SCLC), but ongoing trials are evaluating its use in large cell neuroendocrine carcinoma (LCNEC), neuroendocrine prostate cancer, and other DLL3-expressing malignancies.
What are the common side effects of Tarlatamab?
The most common side effects include cytokine release syndrome (CRS), fatigue, fever, low blood pressure, and immune-related toxicities. Less frequently, neurotoxicity and infections may occur, requiring close monitoring.
How is Tarlatamab administered?
Tarlatamab is given as an intravenous (IV) infusion, typically over one hour using an infusion pump. Patients are closely monitored, especially during the first cycle, as CRS is more common early in treatment.
How should Tarlatamab be stored before administration?
Unopened vials should be refrigerated (2-8°C / 36-46°F) in their original packaging, protected from light. Once prepared, the infusion bag should be used within 8 hours at room temperature or 7 days if refrigerated. The prepared solution should never be re-refrigerated after warming.
Are there any ongoing clinical trials for Tarlatamab?
Yes, several trials are investigating Tarlatamab, including the DeLLphi-301 and DeLLphi-303 trials for ES-SCLC. The DeLLphi-305 Phase 3 trial is exploring its role in first-line maintenance therapy in combination with PD-L1 inhibitors.
Can Tarlatamab be used in combination with other cancer treatments?
Yes, ongoing research is evaluating Tarlatamab in combination with immune checkpoint inhibitors (such as Durvalumab or Atezolizumab) and chemotherapy to improve outcomes in patients with neuroendocrine tumors.
Who should not receive Tarlatamab?
Patients with severe active infections, uncontrolled autoimmune disorders, or a history of severe allergic reactions to similar biologic therapies may not be eligible for Tarlatamab treatment. A full medical evaluation is necessary before starting therapy.
What precautions should patients take during Tarlatamab treatment?
Patients should avoid immunosuppressive medications unless prescribed, monitor for signs of infection or CRS, and report new neurological symptoms immediately. Regular follow-up with an oncology team is essential to manage potential side effects.


