
Maria (Masha) Babak: A significant step forward for advanced HR+ HER2-negative breast cancer
Maria (Masha) Babak, Head of The Babak Lab and an Assistant Professor at the City University of Hong Kong, reshared a post by The Babak Lab on LinkedIn:
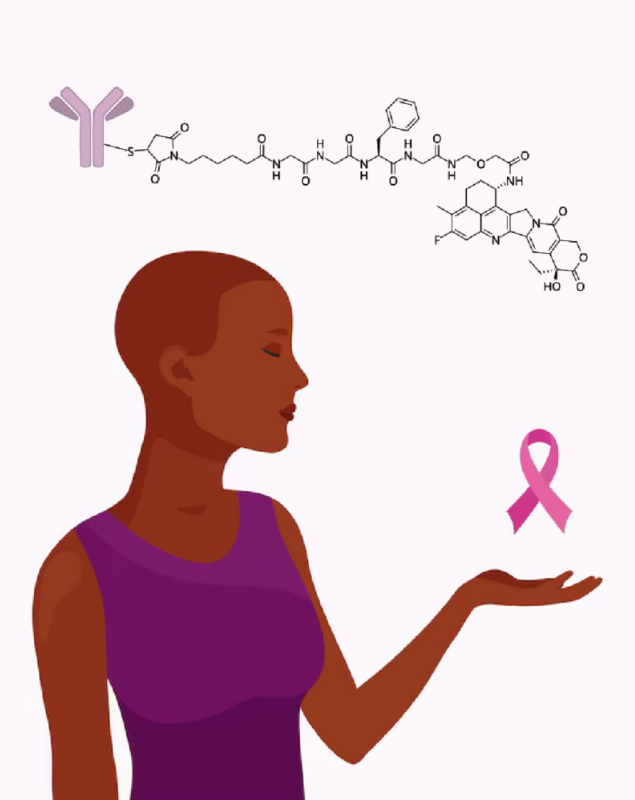
“Thrilled to see the FDA’s approval of datopotamab deruxtecan-dlnk (Datroway) — a significant step forward for patients with advanced HR-positive, HER2-negative breast cancer.
This innovative therapy not only extends progression-free survival but also brings new hope to those who’ve exhausted other options. A testament to the power of research and collaboration in transforming lives.”
Quoting The Babak Lab‘s post:
“Clinical Mondays: Exciting News in Breast Cancer Treatment!
On January 17th, 2025, the FDA approved datopotamab deruxtecan-dlnk (Datroway, Daiichi Sankyo, Inc.), a groundbreaking Trop-2-directed antibody and topoisomerase inhibitor conjugate, for adult patients with unresectable or metastatic, hormone receptor (HR)-positive, HER2-negative breast cancer. This approval marks a significant step forward for patients who have previously received endocrine-based therapy and chemotherapy for advanced disease.
Key findings include:
- Progression-free survival (PFS): 6.9 months with Datroway vs. 4.9 months with chemotherapy.
- Overall survival (OS): 18.6 months with Datroway vs. 18.3 months with chemotherapy (not statistically significant).
- Objective response rate (ORR): 36% with Datroway vs. 23% with chemotherapy.
- Duration of response (DOR): 6.7 months with Datroway vs. 5.7 months with chemotherapy.
While the most common adverse reactions included stomatitis, nausea, and fatigue, the benefits of this innovative therapy offer new hope for patients with limited treatment options. This approval underscores the importance of continued innovation in oncology and the potential of antibody-drug conjugates to transform cancer care.
For more details, the full prescribing information will be available on Drugs@FDA.
Let’s celebrate this advancement and continue working toward better outcomes for patients everywhere.
Source.”
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
