Boehringer Ingelheim and Jazz Pharmaceuticals have launched a strategic clinical collaboration to evaluate a novel combination therapy for HER2-positive breast cancer. The partnership adds a new Phase 1b cohort to Boehringer’s ongoing BEAMion-BCGC1 trial, pairing Boehringer’s oral HER2 inhibitor zongertinib with Jazz’s bispecific antibody zanidatamab in patients with advanced HER2-expressing breast tumors.

You Can Read More About Zongertinib on OncoDaily.

You Can Read More About Zanidatamab on OncoDaily.
HER2 in Breast Cancer: Rationale for Targeted Therapy
HER2 (human epidermal growth factor receptor 2) is a growth-promoting cell-surface protein that is overproduced due to gene amplification in roughly 15–20% of breast cancers. HER2-positive breast cancer is historically aggressive, before targeted treatments, it was associated with rapid progression and poor patient outcomes. Even today, patients with advanced or metastatic HER2-driven tumors face five-year survival rates of only about 50%. The development of HER2-targeted therapies has dramatically improved this outlook by directly attacking a key cancer driver. Blocking HER2’s activity can halt tumor growth and even induce tumor regression, as first demonstrated with the monoclonal antibody trastuzumab (Herceptin) in the late 1990s.
Subsequent therapies that inhibit HER2 signaling have extended survival further. For example, combining two anti-HER2 antibodies (trastuzumab plus pertuzumab) with chemotherapy in the CLEOPATRA trial prolonged median overall survival from ~41 months to ~57 months in metastatic HER2-positive breast cancer. At eight years follow-up, 37% of patients on dual antibody therapy were still alive (Swain et al., 2020) an unprecedented landmark, underscoring how HER2 blockade has altered the natural history of this disease.
Despite these advances, HER2-positive breast cancer remains lethal for many patients. Tumors eventually develop resistance to existing drugs, and metastases (especially to the brain) often evade control. Thus, there is a continued unmet need for new HER2-targeted strategies. The Boehringer–Jazz collaboration arises from this need, aiming to improve outcomes by simultaneously targeting HER2 through two distinct modalities.
Trial Design: Combining Zongertinib and Zanidatamab
The joint Boehringer–Jazz effort integrates these two agents – zongertinib and zanidatamab – into a combination regimen with the goal of dual HER2 blockade. The collaboration will launch a Phase 1b study cohort within Boehringer’s BEAMion-BCGC1 trial to evaluate the safety and preliminary efficacy of this combination in HER2-positive breast cancer patients. BEAMion-BCGC1 is an open-label, multi-center platform trial exploring zongertinib in various HER2-driven cancers and combinations.
In this trial, different patient cohorts receive zongertinib either alone or alongside other HER2-directed treatments, including ADCs like trastuzumab deruxtecan (T-DXd) and trastuzumab emtansine (T-DM1), as well as conventional chemo-antibody therapy (trastuzumab + capecitabine) (ClinicalTrials.gov, NCT06324357). The new collaboration adds a zanidatamab + zongertinib cohort to this lineup, reflecting the companies’ confidence in a two-pronged attack on HER2.
Study objectives and design: In the Phase 1b portion, the primary objective is to determine a recommended combination dose – i.e. the highest dose of zongertinib that can be given with zanidatamab safely (ClinicalTrials.gov, NCT06324357). Patients will receive zongertinib by mouth daily, while zanidatamab is given as periodic infusions, in repeated treatment cycles. Dose-escalation will proceed cautiously, with investigators monitoring for dose-limiting toxicities (such as significant liver enzyme elevations, cardiac effects, or other known risks of HER2 inhibitors ). Once an optimal dosing is identified, an expansion phase (Phase 2) may enroll additional patients to more fully evaluate anti-tumor activity at that dose.
Key endpoints include safety outcomes and tumor response measures. Safety will be assessed by the frequency and severity of adverse events, particularly any severe (grade 3 or higher) events and any signs of overlapping toxicity between the two agents. On the efficacy side, tumor responses will be tracked by imaging (MRI/CT scans) according to RECIST criteria, with objective response rate (proportion of patients achieving significant tumor shrinkage) as a major endpoint (ClinicalTrials.gov, NCT06324357). Duration of response and progression-free survival will also be recorded if the study progresses to expansion cohorts.
The trial aims to generate insights on whether dual blockade of HER2 – attacking the receptor’s external domain with an antibody and its internal signaling with a TKI, can produce deeper or more durable responses than either approach alone. According to the companies, these data could inform future development of combination therapies for HER2-positive cancers if promising.
Notably, this is one of the first studies to combine a HER2-targeted TKI with a bispecific antibody in breast cancer. The scientific premise is supported by prior evidence that multi-faceted inhibition of HER2 can yield synergistic anti-tumor effects. For instance, earlier studies showed that adding a TKI (like lapatinib) to trastuzumab improved tumor suppression compared to the TKI alone in refractory breast cancer (Blackwell at al., 2012). The present trial will explore a similar principle using two cutting-edge agents.
Context in the HER2 Treatment Landscape
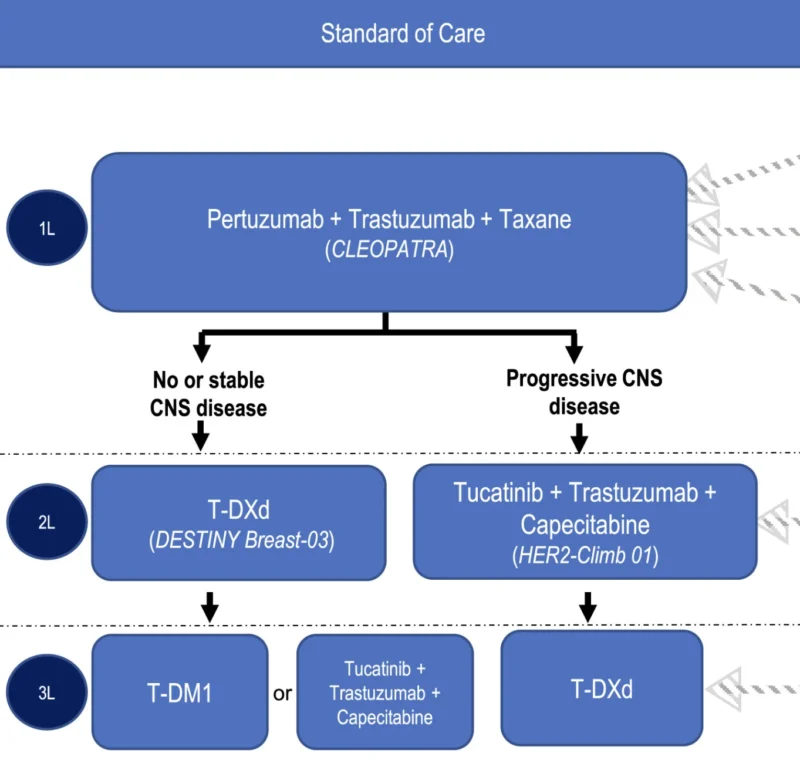
Current Standard of Care in HER2-positive advanced breast cancer from Agostinetto et al,. 2024.
This collaboration unfolds against a backdrop of extensive therapeutic progress in HER2-positive breast cancer – and persistent challenges. Current standard of care for newly diagnosed metastatic HER2-positive breast cancer typically involves dual HER2 antibody therapy (trastuzumab + pertuzumab) plus chemotherapy, which has extended patient survival to a median of nearly 5 years (Swain et al., 2020). After first-line treatment, patients often receive sequential HER2-targeted therapies: for example, the ADC T-DM1 (Kadcyla) has been used in second-line, and more recently, the potent ADC trastuzumab deruxtecan (Enhertu) has demonstrated superior efficacy, achieving prolonged remissions even in some patients whose tumors are resistant to other HER2 drugs.
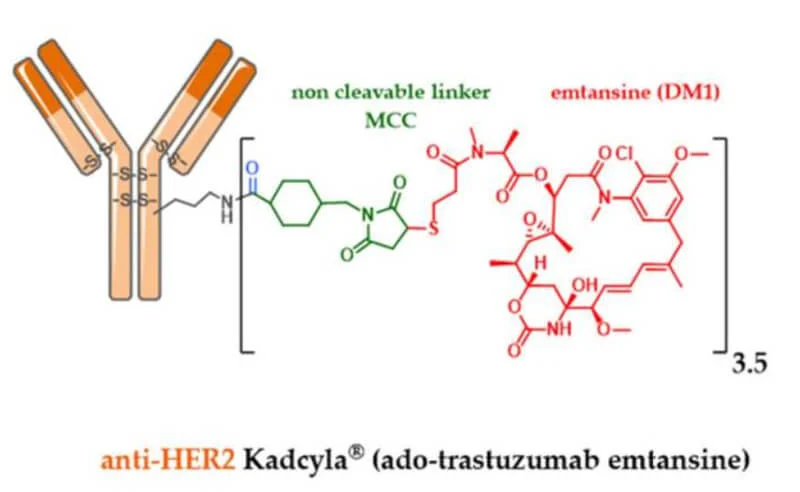
Schematic representation of FDA-approved ADCs: Kadcyla from Joubert et al., 2020.
Small-molecule HER2 inhibitors also play a role; lapatinib (Tykerb) and neratinib (Nerlynx) historically provided incremental benefits, and the newer HER2-selective TKI tucatinib (Tukysa), combined with capecitabine and trastuzumab – has improved outcomes for patients with brain metastases in the HER2CLIMB trial (tucatinib’s addition cut the risk of death and notably helped control brain lesions) (Swain et al., 2020). These advances illustrate a rich arsenal of HER2-directed agents, yet metastatic HER2-positive breast cancer remains incurable. Most patients eventually exhaust available therapies as tumors mutate, bypass HER2 signaling, or metastasize to sites like the brain where treatments are less effective.
The Boehringer Ingelheim–Jazz combination strategy is an innovative response to these unmet needs. By pairing zongertinib and zanidatamab, the approach seeks to shut down HER2 signaling on multiple fronts: inside the cell and at the cell surface. Zongertinib can penetrate tumors (and potentially the brain) to inhibit HER2’s kinase activity, including in cancer cells with HER2 mutations that might not depend on amplification alone. Zanidatamab, meanwhile, locks onto the external receptor, preventing HER2 from activating and flagging the cancer cell for immune attack.
This dual mechanism could, in theory, overcome or delay resistance that arises with single-agent therapy. It is conceptually similar to the successful dual-antibody approach (Herceptin + Perjeta) (Swain et al., 2020), but using an antibody plus a TKI offers a new complementary mode of action. Moreover, each of these agents has shown strong single-agent activity: zongertinib’s ~71% response rate in HER2-mutant cancers and zanidatamab’s efficacy across multiple HER2-positive tumors suggest that combining them is scientifically well-grounded. If this combination demonstrates safety and substantial anti-tumor activity, it could carve out a new treatment option for patients who have progressed on existing HER2 therapies.
In summary, the clinical collaboration between Boehringer Ingelheim and Jazz Pharmaceuticals represents a rational next step in HER2-targeted therapy: combining a cutting-edge HER2 inhibitor with a next-generation antibody to attack an aggressive cancer from dual angles. As the Phase 1b study proceeds, investigators will closely watch safety and efficacy signals. While it is early in development and no outcomes are guaranteed, the scientific rationale and prior data are encouraging. This initiative exemplifies how pharmaceutical collaboration can accelerate the exploration of innovative treatments in the ongoing fight to improve survival for patients with HER2-positive breast cancer.
Written by: Semiramida Nina Markosyan, Editor, OncoDaily Canada


