Zongertinib is a next-generation targeted therapy under investigation for the treatment of cancers driven by specific molecular alterations. Recent 2025 updates have shed light on its therapeutic potential, clinical development, and safety profile. With growing interest in precision oncology, Zongertinib represents a promising addition to the evolving landscape of cancer treatment. This article reviews its current applications, side effects, dosing considerations, and treatment expectations based on the most recent data.
Which company produced Zongertinib?
Zongertinib was developed by Boehringer Ingelheim, a leading global biopharmaceutical company with a growing focus on precision oncology. The drug, also known by its development code BI-1810631 and marketed in the United States as Hernexeos, represents one of Boehringer Ingelheim’s key advances in targeting HER2-mutant non-small cell lung cancer (NSCLC).
In addition to its in-house development, Boehringer Ingelheim has also forged important collaborations to support Zongertinib’s clinical use. In late 2024, the company partnered with Guardant Health to create a companion diagnostic tool, the Guardant360® CDx liquid biopsy assay. This test enables physicians to identify patients with HER2 mutations who may benefit from Zongertinib, allowing for a more precise and personalized approach to treatment.
Through this combination of innovative drug development and strategic partnership, Boehringer Ingelheim is positioning Zongertinib as a significant addition to the evolving landscape of targeted cancer therapies.
How does Zongertinib work?
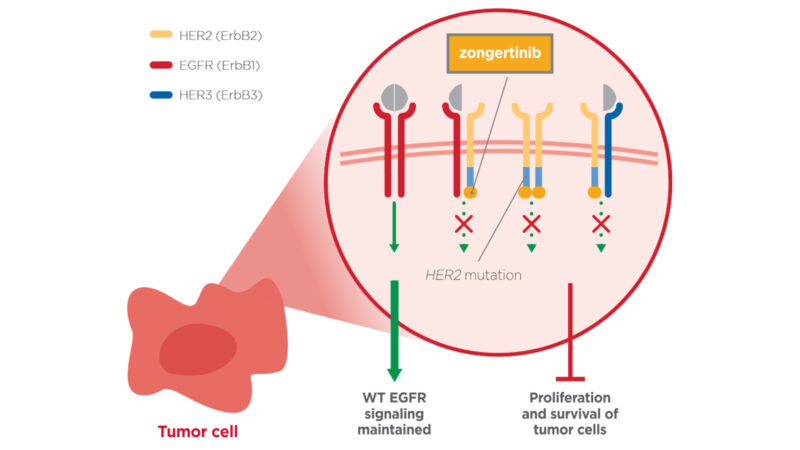
Zongertinib is a selective tyrosine kinase inhibitor (TKI) designed to target cancers driven by HER2 (ERBB2) mutations, particularly HER2 tyrosine kinase domain (TKD) activating mutations.
In certain cancers, including subsets of non-small cell lung cancer (NSCLC), mutations in the HER2 gene lead to abnormal activation of the HER2 receptor, which continuously stimulates cancer cell growth and survival. Traditional HER2-targeted therapies (such as monoclonal antibodies or antibody–drug conjugates) often focus on HER2 overexpression or amplification, but they may be less effective against cancers with HER2 mutations in the kinase domain.
Zongertinib was developed specifically to address this gap. By binding to the mutated HER2 kinase domain, it blocks downstream signaling pathways that drive uncontrolled tumor growth, such as the MAPK/ERK and PI3K/AKT pathways. This selective inhibition disrupts cancer cell proliferation and survival while aiming to minimize effects on normal cells.
Source: Boehringer Ingelheim Official Website
What Cancers Is Zongertinib Approved to Treat?
On August 8, 2025, the FDA granted accelerated approval to zongertinib (Hernexeos, Boehringer Ingelheim) for adults with unresectable or metastatic non-squamous NSCLC carrying HER2 (ERBB2) tyrosine kinase domain mutations, after prior systemic therapy. Alongside the approval, the FDA authorized the Oncomine Dx Target Test as a companion diagnostic to identify eligible patients. Together, these advances mark a significant milestone in expanding treatment options for this rare but challenging subgroup of NSCLC.
What research is behind the approval?
The accelerated approval of zongertinib (Hernexeos, Boehringer Ingelheim Pharmaceuticals, Inc.) on August 8, 2025 was based on the results of the Beamion LUNG-1 trial (NCT04886804). This Phase 2, open-label, multicenter study evaluated patients with unresectable or metastatic non-squamous NSCLC harboring HER2 tyrosine kinase domain (TKD) activating mutations who had received prior systemic therapy.
Results from Beamion LUNG-1 demonstrated meaningful activity. In patients previously treated with chemotherapy but no prior HER2-targeted therapy, the objective response rate (ORR) reached 75%, with 58% maintaining responses for at least six months. Even in patients who had already received HER2 antibody–drug conjugates (ADCs), zongertinib achieved a 44% ORR, with 27% showing responses lasting six months or longer. These findings highlight its potential effectiveness in both treatment-naïve and previously treated HER2-targeted settings.
Zongertinib is a highly selective, irreversible tyrosine kinase inhibitor that directly targets HER2 TKD mutations. By blocking abnormal HER2 signaling that drives tumor growth, it halts cancer progression while minimizing effects on healthy cells. Its precision design allows it to avoid unwanted inhibition of wild-type EGFR, a limitation of earlier HER2-directed therapies.

Find more information about FDA Approval of Zongertinib for HER2 TKD Mutated Non-Squamous NSCLC on OncoDaily.
Zongertinib side effects and its management
As with other targeted therapies, treatment with Zongertinib may be associated with a range of side effects. These reactions can vary in severity, and not every patient will experience them. Understanding the potential adverse effects and their management strategies is an important part of ensuring safe and effective use of the drug.
Common side effects
The most frequent side effects include diarrhea and lowered lymphocyte counts, each affecting around half of patients. Other reactions seen in about one-third to nearly half of patients are decreases in white blood cells, hemoglobin, and platelets, as well as elevated liver enzymes such as ALT and AST.
Skin-related issues, particularly rash and nail changes, are also common. Many patients experience fatigue, nausea, vomiting, or cough. Some laboratory changes like increased triglycerides, bilirubin, and lipase also occur frequently, though they often show up only on routine blood tests.
Less common side effects
Less frequent but still noteworthy effects include decreased left ventricular ejection fraction, which reflects some degree of heart function reduction, and pulmonary embolism, a type of blood clot in the lungs. A small percentage of patients (about 1.2%) developed interstitial lung disease or pneumonitis, which can be serious if not caught early.
Severe laboratory abnormalities were less common but included ALT elevations, dyspnea or shortness of breath, and drug-induced liver injury. Fatal outcomes were rare, with pneumonia reported as a cause of death in approximately 1% of patients.
Management if side effects
Management typically relies on early recognition, supportive care, and careful monitoring. Diarrhea is generally controlled with antidiarrheal agents and adequate hydration, but moderate to severe cases may require temporary interruption of therapy and subsequent dose reduction. Elevated liver enzymes necessitate regular blood tests, with dose adjustments or treatment pauses if significant abnormalities are detected. Skin toxicities can usually be alleviated with moisturizers, topical corticosteroids, or antihistamines, while more severe cases may require treatment interruption.
Hematologic changes are managed with routine laboratory monitoring and, when necessary, supportive measures such as transfusions or growth factors. Serious adverse events, such as interstitial lung disease or cardiac dysfunction, demand immediate medical evaluation. In such cases, permanent discontinuation of Zongertinib is often warranted.

What is the Recommended Dosage of Zongertinib?
Zongertinib is available as oral tablets (60 mg) and is indicated for adults with unresectable or metastatic non-squamous NSCLC harboring HER2 tyrosine kinase domain (TKD) activating mutations, following prior systemic therapy. The recommended starting dose is 180 mg once daily for patients weighing 90 kg or more, and 120 mg once daily for those under 90 kg. Treatment should be continued until disease progression or unacceptable toxicity.
Dose Adjustments for Toxicity
For patients unable to tolerate therapy, stepwise dose reductions are advised. A 180 mg daily regimen may be reduced to 120 mg, then to 60 mg if necessary. Treatment is permanently discontinued if toxicity persists at the lowest tolerated dose. Similarly, patients starting at 120 mg may be reduced to 60 mg, with discontinuation if further reduction is required.
Specific adverse events require tailored management. In cases of left ventricular dysfunction, therapy may be held until recovery, with resumption at the same or a reduced dose depending on severity and recovery time. Symptomatic heart failure necessitates permanent discontinuation. Interstitial lung disease or pneumonitis also requires interruption, with permanent discontinuation for grade 3–4 events or recurrent toxicity. For diarrhea, supportive antidiarrheal therapy is initiated, with dose holds and reductions applied for persistent or severe cases. Other grade 3 toxicities typically lead to dose interruption and reduction upon recovery, while grade 4 reactions require permanent discontinuation.
Read more about Non-Small Cell Lung Cancer: Causes, Symptoms, Diagnosis, Treatment Options, and Latest 2025 Advances in Targeted and Immunotherapy on OncoDaily.
How is Zongertinib Administered?
Zongertinib is taken by mouth and can be swallowed with or without food. The tablets should always be swallowed whole with a glass of water—never split, crushed, or chewed.
If a dose is missed and less than 12 hours have passed, the patient should take it as soon as possible. If more than 12 hours have gone by, the missed dose should be skipped, and treatment should continue with the next scheduled dose. If vomiting occurs after taking Zongertinib, an extra dose should not be taken; instead, the next dose should be taken at the usual time.
The medication should be stored at room temperature (20–25°C) in its original container to protect it from moisture. Temporary exposure to slightly warmer or cooler temperatures (15–30°C) is acceptable. The bottle must be kept tightly closed, with the desiccants left inside. Once the bottle is opened, any unused tablets should be discarded after three months.
What to Avoid During Zongertinib Treatment?
Patients receiving Zongertinib should observe specific precautions to optimize safety and therapeutic effectiveness. Grapefruit, Seville oranges, and their juices should be avoided, as these can inhibit hepatic enzymes involved in Zongertinib metabolism, potentially increasing systemic exposure and the risk of adverse effects.
Concomitant use of medications or supplements that strongly induce or inhibit CYP3A4 may alter Zongertinib plasma concentrations, reducing efficacy or increasing toxicity. It is essential for patients to provide a comprehensive list of prescription, over-the-counter, and herbal products to their healthcare team before initiating therapy.
Excessive alcohol consumption should be minimized due to the potential for additive hepatotoxicity. Zongertinib is contraindicated during pregnancy and breastfeeding because of the risk of fetal harm and potential transfer into breast milk.
Patients experiencing treatment-related dizziness, fatigue, or visual disturbances should refrain from driving or operating machinery until these effects resolve. Adherence to these precautions supports the safe administration of Zongertinib and helps maximize clinical benefit while minimizing the risk of serious complications.
Metabolism and Half-Life of Zongertinib
The majority of metabolism occurs via CYP-mediated oxidation, mainly through CYP3A4 and CYP3A5 enzymes, accounting for approximately half of its hepatic breakdown. Additional metabolism occurs through glucuronidation, largely mediated by UGT1A4, and through glutathione conjugation, each contributing to a smaller portion of overall metabolism.
The drug has a plasma half-life of around 12 hours, with a clearance rate of approximately 115 mL per minute. Following a single oral dose, most of the drug is excreted in the feces, with roughly one-third eliminated unchanged. Only a small fraction is recovered in the urine, indicating that renal elimination plays a minor role in its overall clearance.
Zongertinib effectiveness over time
The clinical benefit of Zongertinib in patients with HER2 tyrosine kinase domain–mutant non-squamous NSCLC is reflected in both response rates and the durability of those responses. In the pivotal Beamion LUNG-1 trial, patients who had previously received systemic therapy demonstrated meaningful tumor shrinkage shortly after starting treatment. The median duration of response was notable, with a substantial proportion of patients maintaining tumor control for six months or longer, particularly in those who were naïve to prior HER2-targeted therapies.
Response to Zongertinib tends to be sustained in many patients, though progression may eventually occur as tumors develop resistance mechanisms over time. Continuous monitoring through imaging and clinical assessment is critical to evaluate ongoing benefit and to determine the appropriate duration of therapy. Early intervention for adverse effects or dose adjustments can help patients maintain therapy and maximize the period of clinical response.
Overall, Zongertinib demonstrates robust early activity, with durable responses that can be maintained over months, providing an important targeted option for this genetically defined NSCLC population.

Read more about the FDA granting Breakthrough Therapy Designation to Zongertinib (HERNEXEOS) for first-line treatment of HER2-mutant advanced NSCLC on OncoDaily.
Ongoing trials with Zongertinib
The Beamion BCGC-1 trial (NCT06324357) is a Phase Ib/II study evaluating the safety, tolerability, and effectiveness of Zongertinib in adults with advanced HER2-positive cancers, including metastatic breast, gastric, gastroesophageal junction, and esophageal adenocarcinomas.
The study tests Zongertinib alone and in combination with trastuzumab deruxtecan, trastuzumab emtansine, or trastuzumab plus capecitabine.
It aims to identify the optimal dose and assess whether the drug can shrink tumors in patients whose prior treatments were unsuccessful. Participants receive treatment in cycles and are monitored with imaging and clinical evaluations. The trial began in June 2024, plans to enroll about 582 patients, and is expected to finish in September 2029.
The Beamion PANTUMOR-1 trial (NCT06581432) is a Phase II, multicenter study evaluating the safety and effectiveness of Zongertinib in adults with advanced cancers harboring HER2 alterations, particularly in patients whose prior treatments were unsuccessful.
Participants are assigned to multiple groups based on cancer type and specific HER2 alterations, receiving a daily dose of Zongertinib. Treatment continues as long as patients benefit and can tolerate the therapy.
Throughout the study, participants undergo regular assessments, including imaging to monitor tumor size and spread, as well as routine evaluations to track safety and any adverse effects.
FAQ
What is Hernexeos used for?
Zongertinib is approved for adults with unresectable or metastatic non-squamous NSCLC carrying HER2 tyrosine kinase domain (TKD) mutations after prior systemic therapy.
How does Zongertinib work?
It is a selective tyrosine kinase inhibitor that blocks abnormal HER2 signaling caused by HER2 mutations, stopping cancer cell growth and survival.
What are the common side effects of Zongertinib?
Diarrhea, fatigue, rash, low blood counts, and elevated liver enzymes are among the most frequent side effects reported in clinical trials.
How is Zongertinib taken?
Zongertinib is an oral tablet taken once daily, with dosing based on body weight. Tablets must be swallowed whole, with or without food.
Are there foods or drugs to avoid while taking Zongertinib?
Patients should avoid grapefruit, Seville oranges, and strong CYP3A4 inducers or inhibitors, which can affect drug levels in the body.
What is the half-life of Zongertinib?
Zongertinib has a plasma half-life of about 12 hours. It is mainly metabolized by CYP3A4/5 enzymes, with most of the drug excreted in feces.


