In August 8, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to zongertinib (brand name Hernexeos, Boehringer Ingelheim Pharmaceuticals, Inc.) for the treatment of adults with unresectable or metastatic non-squamous non-small cell lung cancer (NSCLC) whose tumors harbor HER2 (ERBB2) tyrosine kinase domain (TKD) activating mutations, as detected by an FDA-approved test. Eligible patients must have previously received systemic therapy.
As part of this approval, the FDA also authorized the Oncomine Dx Target Test (Life Technologies Corporation) as a companion diagnostic to identify HER2 TKD mutations in patients with non-squamous NSCLC who may benefit from treatment with zongertinib. This marks an important milestone in targeted therapy for lung cancer, providing a new option for a rare but challenging patient subgroup.
Beamion LUNG-1: The Trial Behind the Approval
The FDA decision was based on results from Beamion LUNG-1 (NCT04886804), an open-label, multi-center, multi-cohort Phase 2 trial evaluating the efficacy and safety of zongertinib in patients with unresectable or metastatic non-squamous NSCLC and confirmed HER2 TKD mutations who had received prior systemic treatment.
The primary endpoints were:
- Objective Response Rate (ORR) – the proportion of patients with tumor size reduction
- Duration of Response (DOR) – the length of time the tumor remains responsive
Efficacy assessments were performed by blinded independent central review using RECIST v1.1 criteria.
Key Efficacy Findings
The trial enrolled patients into two major efficacy cohorts:
HER2 TKI/ADC-naïve cohort
71 patients previously treated with platinum-based chemotherapy but without prior HER2-targeted tyrosine kinase inhibitors or antibody-drug conjugates (ADCs)
- ORR: 75% (95% CI: 63–83)
- DOR ≥ 6 months: 58% of patients
Post-HER2 ADC cohort
34 patients previously treated with both platinum-based chemotherapy and a HER2-targeted ADC
- ORR: 44% (95% CI: 29–61)
- DOR ≥ 6 months: 27% of patients
These results underscore zongertinib’s potential to deliver meaningful responses in both treatment-naïve and previously treated HER2-targeted settings.
Safety Profile
The safety information for zongertinib includes warnings for:
- Hepatotoxicity
- Left ventricular dysfunction
- Interstitial lung disease/pneumonitis
- Embryo-fetal toxicity
The recommended dose is weight-based:
- < 90 kg: 120 mg orally once daily
- ≥ 90 kg: 180 mg orally once daily
Zongertinib may be taken with or without food and should be continued until disease progression or unacceptable toxicity.
Find more information on FDA Official Website.
What is Zongertinib and How does it work?
Zongertinib (Hernexeos) is an oral, targeted cancer therapy developed by Boehringer Ingelheim Pharmaceuticals, Inc. It is designed for patients with non-squamous non-small cell lung cancer (NSCLC) whose tumors carry HER2 (ERBB2) tyrosine kinase domain activating mutations, a genetic alteration that drives cancer cell growth. These mutations cause the HER2 protein to remain constantly active, sending continuous growth and survival signals that allow cancer cells to multiply unchecked.
Zongertinib exerts its therapeutic effect by selectively binding to the mutated HER2 kinase, effectively blocking its enzymatic activity and interrupting the downstream oncogenic signaling pathways. This precise mechanism halts tumor progression in cancers reliant on HER2 TKD activating mutations, while minimizing off-target effects on healthy cells.
As a precision medicine, zongertinib offers a promising treatment option for patients whose tumors harbor these specific HER2 mutations, particularly in cases where prior therapies have failed.
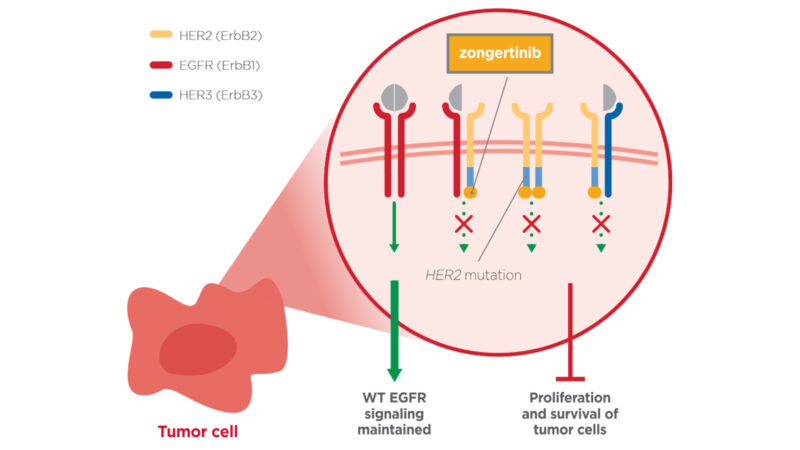
Source: Boehringer Ingelheim Official Website
NSCLC
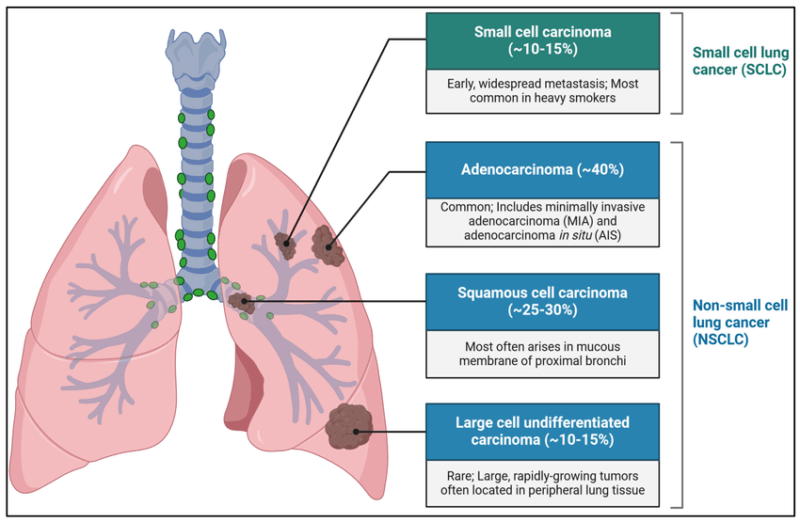
Non-small cell lung cancer accounts for approximately 85% of all lung cancer cases. Compared to small cell lung cancer, NSCLC grows more slowly and is less likely to spread early, offering more treatment options and better prognosis when caught early.
Types of NSCLC
- Adenocarcinoma is the most common, often seen in non-smokers and women. It usually arises in the lung’s outer areas and often involves mutations like EGFR and ALK, which can be targeted by modern therapies.
- Squamous cell carcinoma is linked strongly to smoking and typically develops near the lung’s central airways.
- Large cell carcinoma is less common and usually more aggressive, often diagnosed at later stages.
Rare subtypes, such as adenosquamous and sarcomatoid carcinoma, also exist but are less frequent.
Molecular Subtypes and Their Importance
Advances in molecular profiling have revolutionized NSCLC treatment. Identifying mutations like EGFR, ALK, ROS1, BRAF, and KRAS allows doctors to tailor targeted therapies that improve patient outcomes. For example, EGFR mutations respond well to tyrosine kinase inhibitors, while ALK rearrangements benefit from ALK inhibitors. Immune biomarkers like PD-L1 also help decide if immunotherapy is suitable.
Symptoms and Diagnosis
NSCLC symptoms develop gradually and include persistent cough, chest pain, shortness of breath, and coughing up blood. Because early symptoms can be vague, many cases are detected late. Diagnosis involves imaging tests like CT scans, followed by biopsy and molecular testing to determine the cancer type and genetic profile.
Treatment Options
Treatment depends on the cancer stage and molecular characteristics. Early-stage NSCLC often involves surgery or stereotactic radiation, while locally advanced cases may receive combined chemotherapy and radiation. Advanced NSCLC treatments increasingly rely on targeted therapies and immunotherapies based on genetic testing results. For patients without targetable mutations, immune checkpoint inhibitors have improved survival.
Latest Advances and Ongoing Research
2025 continues to bring new therapies like bispecific antibodies and mRNA vaccines targeting NSCLC. Clinical trials are exploring novel drugs for patients resistant to current treatments, promising better outcomes in the near future.
Read more about Non-Small Cell Lung Cancer: Causes, Symptoms, Diagnosis, Treatment Options, and Latest 2025 Advances in Targeted and Immunotherapy on OncoDaily.


