The European Lung Cancer Congress (ELCC 2025) takes place in Paris from March 26-29, 2025, bringing together leading experts in thoracic oncology. Designed for medical oncologists, radiation oncologists, thoracic surgeons, pneumologists, interventional radiologists, and pathologists, ELCC offers a comprehensive program focused on advancing lung cancer research and improving clinical practice.
Many physicians and organizations have shared posts from Day 2 of ELCC 2025.
ESMO:
“Clinical trials presented at ELCC25 evaluated the efficacy and safety of new treatments for advanced/metastatic RAS-mutated NSCLC.
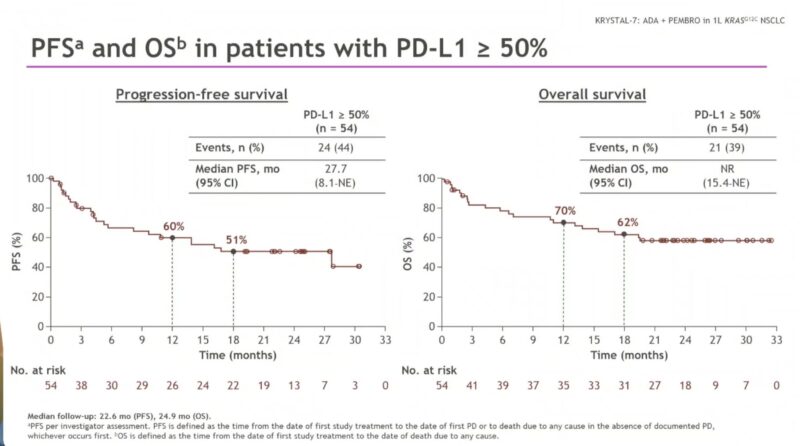
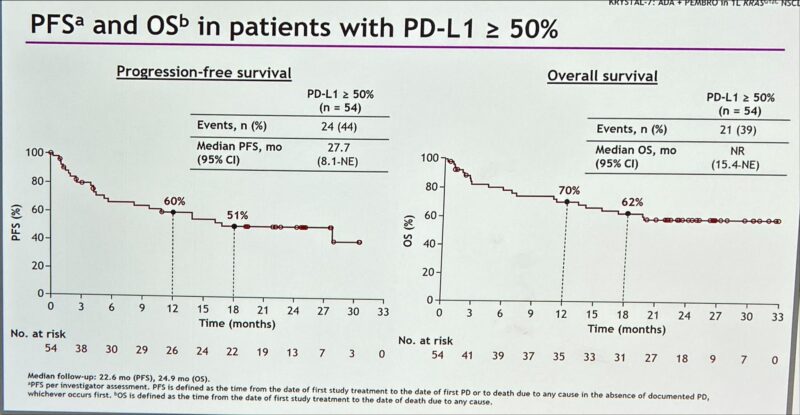
According to updated data from the KRYSTAL-7 phase II trial, adagrasib plus pembrolizumab continued to have encouraging efficacy in patients with KRASG12C-mutated NSCLC and PD-L1 ≥50%. Median progression-free survival (PFS) was 27.7 months but high rates of grade ≥3 adverse events were noted.
In an updated analysis from the KROCUS phase II trial investigating fulzerasib plus cetuximab in untreated patients with KRASG12C-mutated NSCLC, the median PFS was 12.5 months with consistent results across levels of PD-L1 expression and manageable toxicity.
Promising phase I/Ib trial findings were also presented for daraxonrasib in previously treated RAS-mutated NSCLC.
Read the full news article here.”
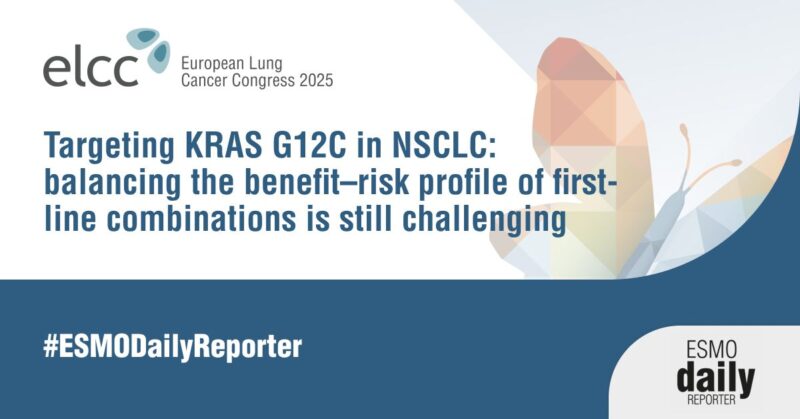
“Taletrectinib vs crizotinib in ROS1 positive NSCLC: a matching-adjusted indirect comparison.
Taletrectinib ORR is 88-90%.
52% reduction in the risk of PD or death and a 66% reduction in the risk of death with taletrectinib vs crizotinib.”
“As always, an insightful presentation by Umberto Malapelle.
‘From single gene testing to CGP’ at ELCC25.
We must continue this path to improve treatment selection!”

“NOW OUT at ELCC25!
COCOON: Preventing Moderate to Severe Dermatologic Adverse Events in First-line EGFR-mutant Advanced NSCLC Treated with Amivantamab Plus Lazertinib.
- AEs ≥G2: OR 0.19 (95% CI, 0.09–0.40)
- NCT06120140.”
“ELCC25 Mini Orals.
Phase II AMAZE-Lung Trial: 2L ami-laz-bev post 3rd generation TKI by Ross Soo.
- 61 patients
- ORR at 12 weeks – 33%
- mDOR 13.9m, mPFS 10.9m, mOS 15.5m
An example of targeting VEGF in EGFR+ NSCLC, another chemo-free approach ”

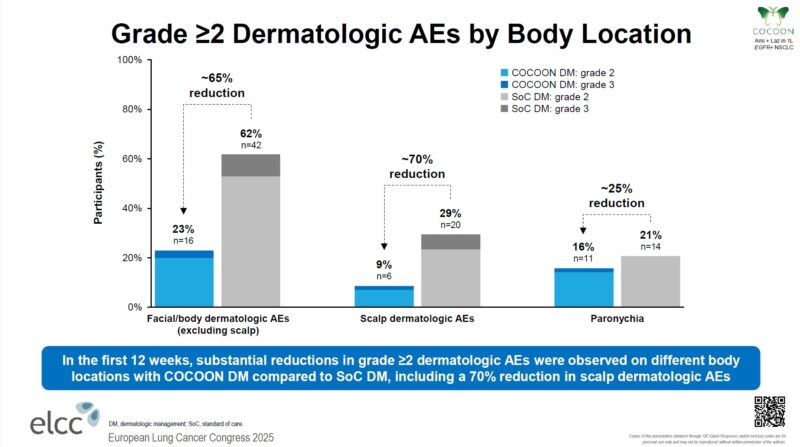
“Randomized phase 2 trial of tobemstomig (bi-specific PD1xLAG3) + platinum-based chemotherapy vs pembrolizumab + chemotherapy in 1L NSCLC. Presented by Ernest Nadal.
Negative trial not supporting LAG-3 combination in NSCLC.”
“1L Adagrasib + pembrolizumab for patients with aNSCLC KRAS G12Cmut and PD-L1 ≥50% in KRYSTAL-7 Phase 2 trial.
- ORR – 59%, DCR – 81%
Median PFS – 27.7m
Median OS NR is 62% at 18m”
ESMO:
“As presented at ELCC25, subcutaneous (SC) pembrolizumab – consisting of pembrolizumab co-formulated with berahyaluronidase alfa – demonstrated noninferiority in pharmacokinetics compared with intravenous (IV) pembrolizumab in the phase III MK-3475A-D77 trial. Similar efficacy outcomes were observed.
Patients may prefer SC immunotherapy administration and other potential benefits include reductions in pharmacy preparation, nursing administration time, infusion-chair occupancy, and infusion-related adverse events than with IV administration.
Results plus commentary in the ESMO Daily Reporter.”

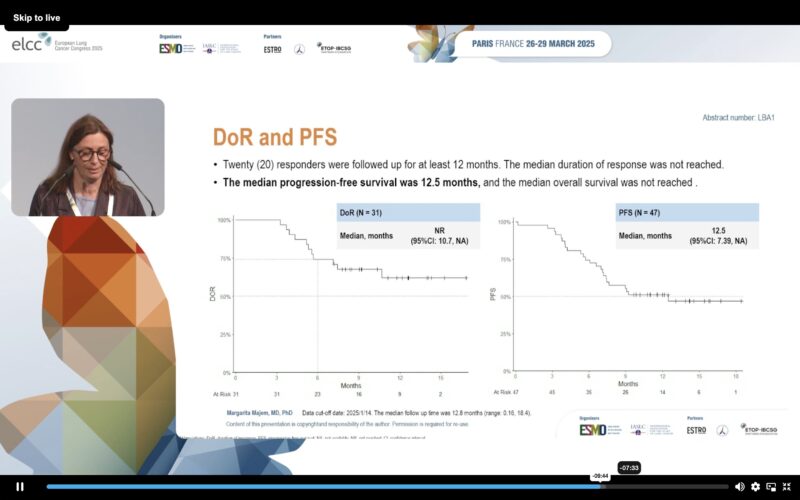
” KROCUS study by Dr. Margarita Majem.
Phase 2 of 1L fulzerasib + cetuximab in KRAS G12C NSCLC:
- Confirmed ORR 68.9%
- +CNS activity
- mPFS 12.5 months.”
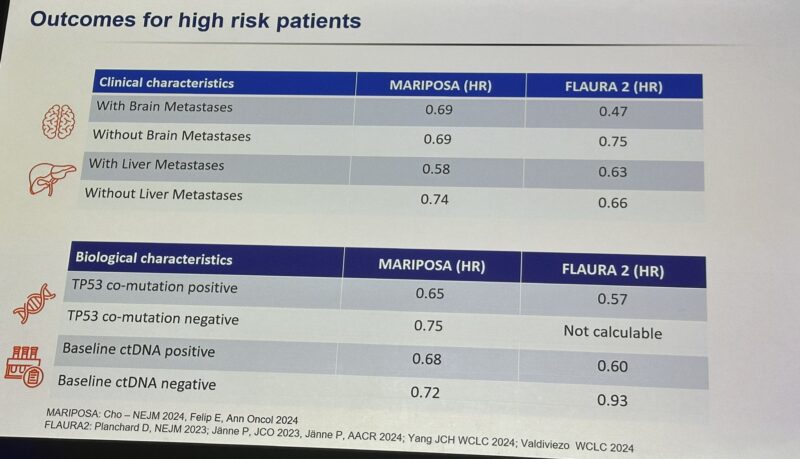
“An excellent debate here at ELCC25 on the optimal choice for 1st Line treatment of EGFR pos NSCLC.
Osimertinib monotherapy vs Combinations (FLAURA2/MARIPOSA)
Insightful lectures by Zosia Piotrowska and Antonio Passaro, focusing on efficacy endpoints, biology, toxicity, and cost-effectiveness. The winner is always the consideration of genetic and clinical risk factors, individualized discussion, and personalized approach.”
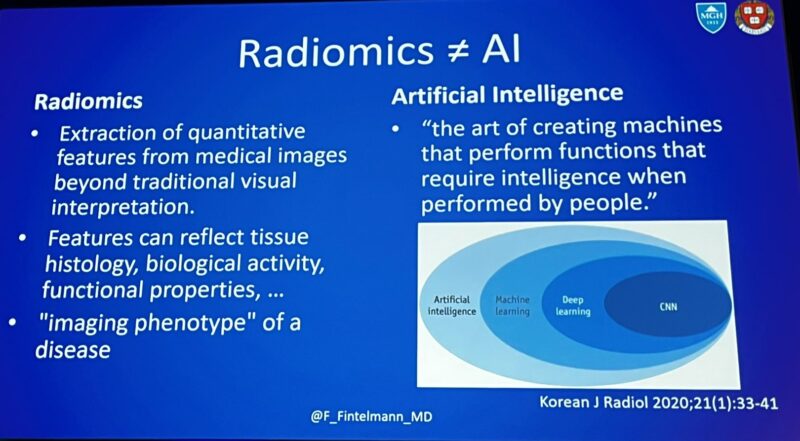
“AI in lung cancer.
Um… so one of the best talks at ELCC, is from a radiologist? What?
Florian Fintelmann from Harvard University outlines the promise of AI in lung imaging:
- Radiomics
- AI model development implementation
- Sybil/lung cancer screening.”
“So this is the situation in Paris tonight after high OS MARIPOSA trial and low toxicity within COCOON trial presentations.
Choose your path.”

“I have many thoughts (surprise), but ultimately, there isn’t a one-size-fits-all treatment. It’s unsettling that opinions are being formed already based on side effects without seeing the COCOON data that will be presented today.
I am a passionate and vocal advocate for QOL, but we can’t ignore the survival data. This isn’t about gaining a few extra months’ – 12+ months is significant and should not be overlooked!
I can’t help but think personally about:
1. My dad would have lived to see me graduate 8th grade (he died a few weeks before), maybe even high school.
2. My mom would have lived to see the birth of my daughter (she died 6 months before) & likely all of my children.
That MATTERS, too!
While I can’t say what my parents would have chosen, I it is ultimately the patient’s/family’s choice! Every family deserves the information and opportunity to discuss ALL options (Informed decision-making). This respect for patient choice should be a priority.
I also feel strongly that the COCOON data should have been presented alongside the MARIPOSA data. It’s just as crucial (even more) because it addresses the significant barrier of side effects that impact whether a patient can stay on treatment. Kudos to J&J for listening to what’s important to patients and developing a study to address the side effects up front!
If QOL truly matters, then so should the data that addresses it! Presenting them together tells a much bigger story and would have changed the conversation. I implore researchers, clinicians, and conference organizers to think about this going forward.”
“The so well deserved award to Prof. Keith Kerr, a mentor to molecular pathology to so many of us, exemplifies the need to emphasize the role of pathology in the optimal management of thoracic malignancies. The Pathologist is our ‘eyes’ to the tumor and much more, to molecular profile leading to personalized treatment.”

“Next talk in liquid biopsy in NSCLC by Thomas John.
Applications of liquid biopsies in oncogene+ NSCLC:
- It can be used to detect dynamic changes in tumor clones
- Future directions: optimize platform selection, fragment omics, clinical trials.”

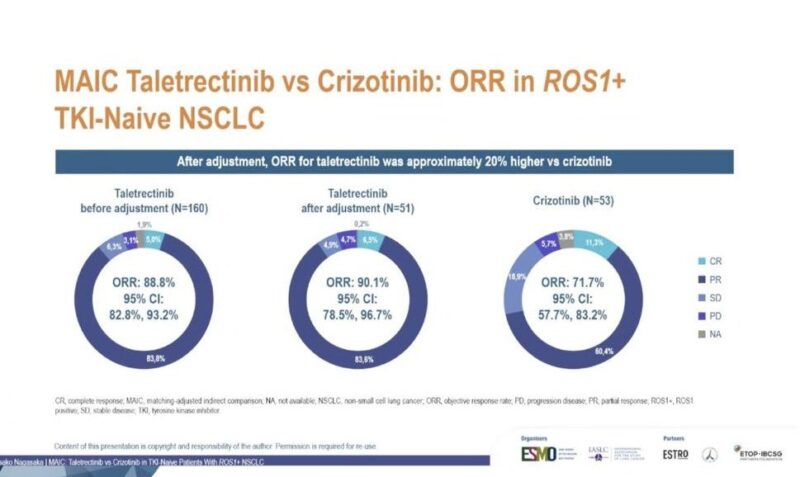
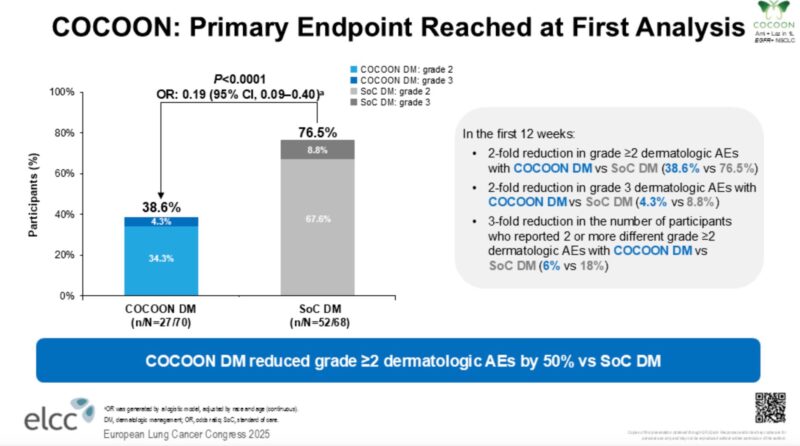
“COCOON data at ELCC25 — can prophylactic enhanced dermatologic management prevent moderate to severe dermatological AEs with amivantamab + lazertinib?
COCOON reaches the primary endpoint.
Within the first 12 weeks,
- G2+ dermatologic AEs decreased from 76.5% to 38.6%.
- G3 dermatologic AEs decreased from 8.8% to 4.3%.
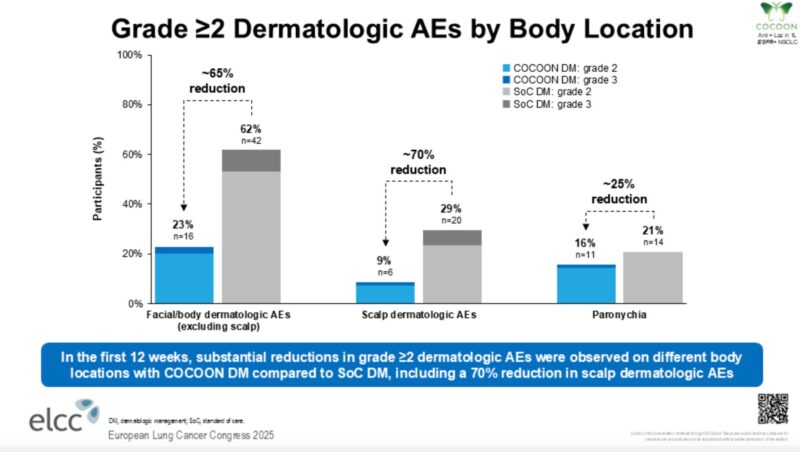
Meaningful slide—scalp lesions remain one of the most challenging dermatologic AEs with this combination.
Encouraging to see a ~70% reduction in G2+ scalp dermatologic AEs (29% v 9%), though small numbers.
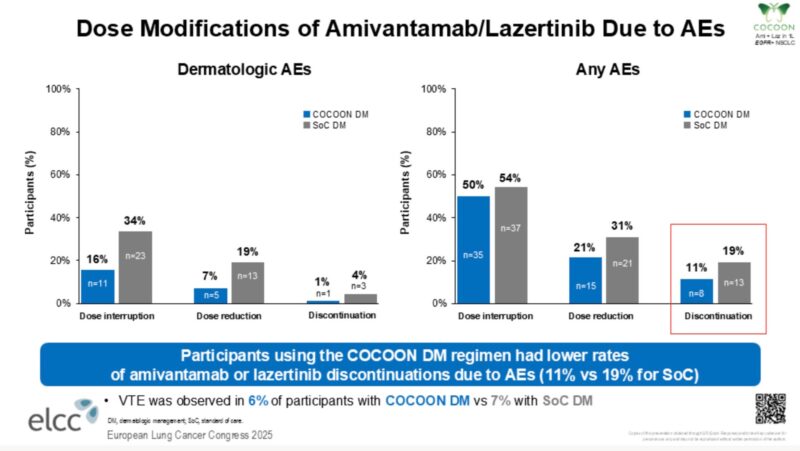
Encouraging to see that patients who received prophylactic-enhanced dermatologic management had lower AE-related discontinuation rates (11% v 19%).
COCOON and MARIPOSA OS data, my thoughts:
- The hand that writes MARIPOSA must write a dermatology consult and the prophylactic COCOON regimen.
- Supportive care can effectively keep patients on treatment and is essential to fully realize the benefit we aim for. “
“Adagrasib 400 mg + Pembrolizumab impressive mPFS in KRAS G12C mutant NSCLC and PDL1 >50% and good safety.
Better than IO or CT+IO alone. Good chemo-free strategy for patients.
Await for phase 3 trial.”
Read OncoDaily’s summary of the MARIPOSA trial.


