PP2R1A mutations predict improved outcomes with immune checkpoint blockade (ICB) in ovarian clear cell carcinoma. In a recent clinical and translational study, patients with PPP2R1A-mutant tumors showed markedly longer survival and stronger immune activation under dual ICB therapy. These findings position PPP2R1A as a promising biomarker and therapeutic target, with implications extending beyond ovarian cancer. The research, led by a team from MD Anderson Cancer Center and AstraZeneca, was published in Nature Medicine in 2025, offering new insights into how genetic mutations can guide more effective cancer treatments.
What Are PPP2R1A Mutations?
PPP2R1A is a gene that encodes the Aα scaffold subunit of protein phosphatase 2A (PP2A), a key enzyme complex that regulates cell growth, survival, and DNA repair.
The PP2A complex is made of three parts — a catalytic subunit, a regulatory subunit, and a scaffold subunit (encoded by PPP2R1A). The scaffold subunit is like a “frame” that holds the other two pieces together, ensuring the enzyme functions properly to control cell signaling.
Most clinically relevant PPP2R1A mutations are missense mutations at “hotspot” sites — especially R183 (R183W, R183Q) and less commonly S256F or W257G. These mutations disrupt how the scaffold binds to the regulatory subunits, resulting in loss of normal PP2A function.
Impact in cancer
- This loss-of-function increases abnormal signaling (e.g., PI3K/AKT pathway activation), contributing to tumor growth.
- Paradoxically, tumors with these mutations also become more immunogenic — they show stronger interferon signaling, form tertiary lymphoid structures, and attract CD8+ T cells and NK cells.
- That’s why in ovarian clear cell carcinoma and other cancers, patients with PPP2R1A mutations respond better to immune checkpoint blockade (ICB)
Study Overview: PPP2R1A mutations portend improved survival after cancer immunotherapy

Authors: Yibo Dai, Anne Knisely, Mitsutake Yano, Minghao Dang, Emily M. Hinchcliff, Sanghoon Lee, Annalyn Welp, Manoj Chelvanambi, Matthew Lastrapes, Heng Liu, Zhe Yuan, Chen Wang, Hao Nie, Stephanie Jean, Luis J. Montaner, Jiakai Hou, Ami Patel, Shrina Patel, Bryan Fellman, Ying Yuan, Baohua Sun, Renganayaki Krishna Pandurengan, Edwin Roger Parra Cuentas, Joseph Celestino, Yan Liu, Jinsong Liu, R. Tyler Hillman, Shannon N. Westin, Anil K. Sood, Pamela T. Soliman, Aaron Shafer, Larissa A. Meyer, David M. Gershenson, David Vining, Dhakshinamoorthy Ganeshan, Karen Lu, Jennifer A. Wargo, Weiyi Peng, Rugang Zhang, Linghua Wang & Amir A. Jazaeri
Background
Ovarian clear cell carcinoma (OCCC), a rare and aggressive histologic subtype, has shown slightly better responses to ICB compared with high-grade serous ovarian cancer, yet outcomes remain poor. Biomarkers such as PD-L1 expression, tumor mutational burden, BRCA status, and homologous recombination deficiency have not reliably predicted ICB benefit in ovarian cancer. This study investigated whether PPP2R1A mutations, encoding the scaffold subunit of the PP2A phosphatase complex, may serve as predictive markers of ICB sensitivity in OCCC and beyond.
Methods and Study Design
The study prospectively analyzed 34 patients with platinum-resistant OCCC treated with dual ICB—primarily tremelimumab (anti-CTLA-4) plus durvalumab (anti-PD-L1), with one case treated with ipilimumab plus nivolumab. Median age was 56 years, and patients had received a median of two prior therapies. Tumor samples underwent next-generation sequencing, RNA sequencing, and multiplexed spatial immune phenotyping.
Clinical outcomes were assessed using Kaplan–Meier analyses for overall survival (OS) and progression-free survival (PFS). Findings were validated in external immunotherapy cohorts, patient-derived xenografts (PDXs), and preclinical models including CRISPR-engineered cell lines and syngeneic mouse models. The study was conducted across multiple levels, integrating clinical, molecular, and preclinical data. The primary clinical cohort included patients with ovarian clear cell carcinoma who received dual immune checkpoint blockade within two investigator-initiated trials (NCT03026062 and NCT01928394).
Tumor samples from these patients underwent comprehensive molecular analyses to identify somatic alterations, including PPP2R1A, ARID1A, AKT, and PIK3CA mutations, alongside evaluation of immune phenotypes. To validate these findings experimentally, preclinical models were developed using PPP2R1A-modified cell lines, CAR-T cell co-culture assays, patient-derived xenograft models in humanized mice, and murine syngeneic ovarian clear cell carcinoma systems.
Finally, cross-cancer validation was performed using large-scale datasets from cBioPortal, encompassing 1,661 patients treated with immune checkpoint blockade and an additional 7,564 patients who had undergone other systemic therapies.
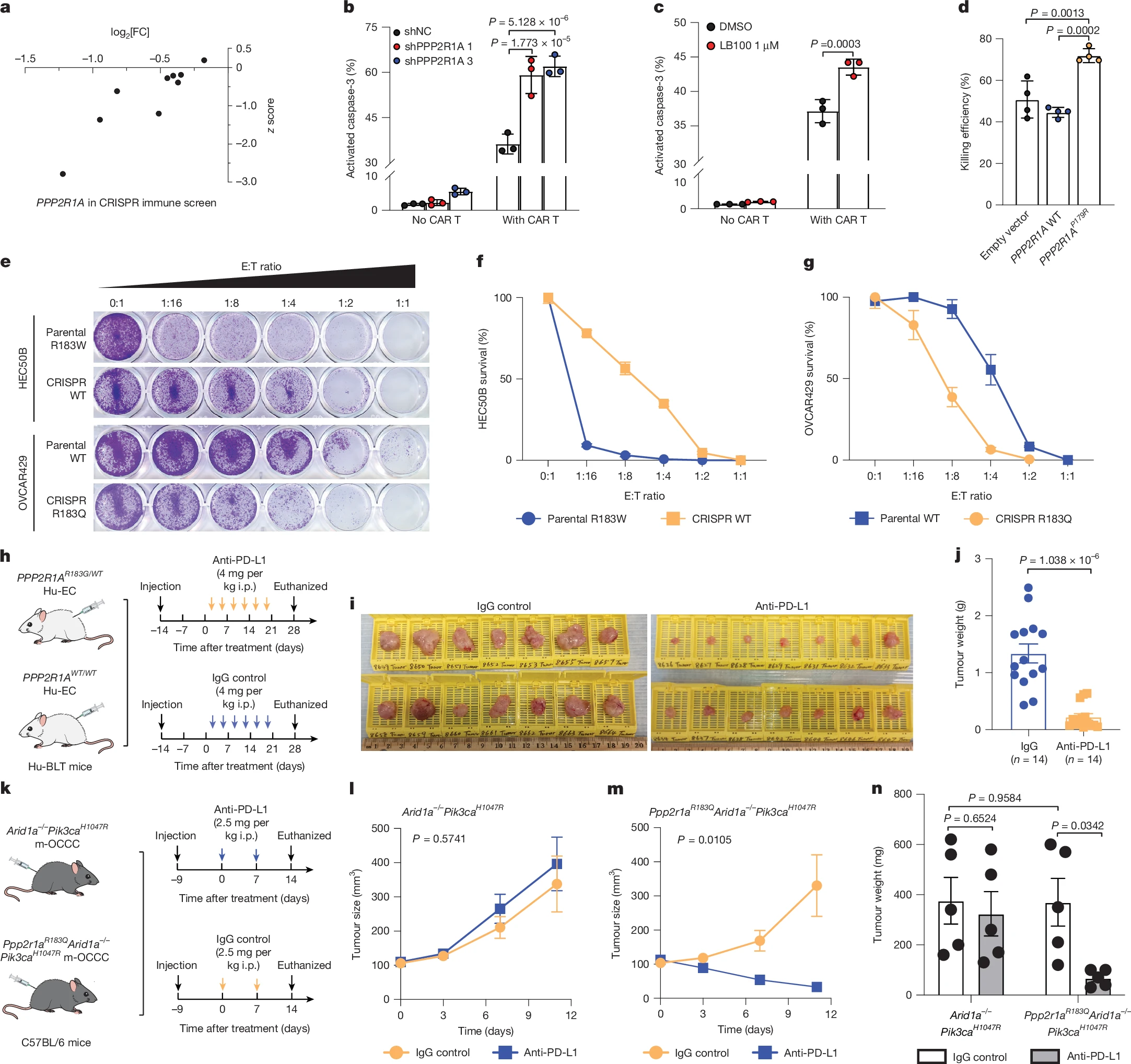
Results of The Study
- Mutation prevalence: 32.4% (11/34) of OCCC patients had PPP2R1A mutations, most at hotspot codon R183.
Survival outcomes
- Median OS for PPP2R1A-mutant OCCC was 66.9 months vs 9.2 months in wild-type (HR 0.40, P=0.031).
- Median PFS was 3.0 months vs 1.8 months (P=0.034).
- Three exceptional responders with PPP2R1A or AKT mutations lived >5 years after ICB.
Translational findings
- PPP2R1A-mutant tumors showed baseline IFNγ enrichment and formation of tertiary lymphoid structures (TLSs).
- After ICB, increased infiltration of CD8+ T cells, activated NK cells, and expansion of CD45RO+ memory CD8+ T cells were observed.
- Higher incidence of grade ≥3 immune-related adverse events in PPP2R1A-mutant patients (45.5% vs 13%).
Preclinical models
- PPP2R1A-mutant or knockdown cells were more sensitive to CAR-T cell killing.
- PP2A inhibitor LB-100 enhanced immunotherapy efficacy in vitro and in vivo.
- PDX models with PPP2R1A mutations responded to anti-PD-L1 therapy, while wild-type tumors did not.
Cross-cancer validation
- In a cohort of 1,661 ICB-treated patients, PPP2R1A mutations correlated with longer OS (not reached vs 18 months, P=0.033).
- No benefit was seen in patients treated with non-ICB therapies.
- Similar findings were observed in endometrial cancer patients treated with pembrolizumab plus lenvatinib, particularly in TP53-mutated subgroups.
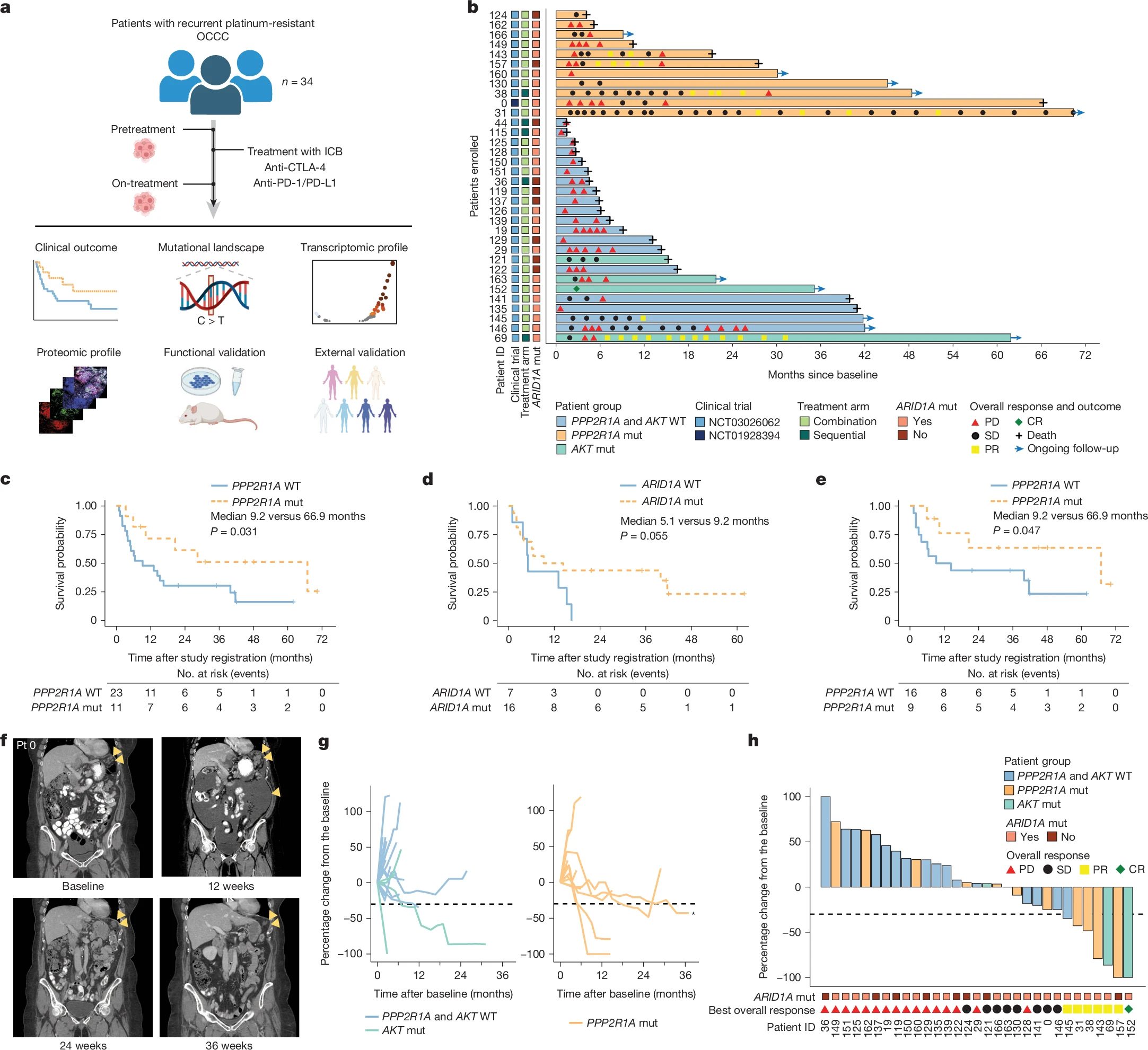
Key Takeaway Messages
- PPP2R1A mutations represent a novel predictive biomarker for ICB efficacy, with potential applications across multiple tumor types.
- Pharmacologic PP2A inhibition may mimic the immune-sensitizing effect of PPP2R1A mutations, providing a therapeutic opportunity even in wild-type patients.
- Integration of PPP2R1A testing could refine patient selection for immunotherapy in ovarian and endometrial cancers.
- Ongoing trials combining LB-100 with ICB (NCT06065462) will clarify the translational potential of this approach.
This study identifies loss-of-function PPP2R1A mutations as a significant determinant of prolonged survival after immune checkpoint blockade in ovarian clear cell carcinoma and potentially other cancers. By enhancing tumor immunogenicity and reshaping the immune microenvironment, PPP2R1A mutations act as predictive biomarkers for immunotherapy response. Importantly, pharmacological inhibition of PP2A may replicate these effects, offering a promising strategy to overcome resistance to ICB and expand benefit to a broader population of patients.
George L. Kumar, PhD, MBA, Senior Director at AstraZeneca (US Medical Affairs – Oncology), shared on his LinkedIn
“PPP2R1A may emerge as a novel therapeutic target and predictive biomarker to guide immunotherapy strategies.”
Overview of Ovarian Clear Cell Carcinomas
Clear cell carcinoma of the ovary (OCCC) is a relatively rare subtype of epithelial ovarian cancer, representing about 5 to 10 percent of cases in Western countries and up to 20 percent in East Asian populations. It is named for the clear, glycogen-rich appearance of the cancer cells under the microscope. OCCC is strongly associated with endometriosis and may develop from endometriotic cysts, also known as endometriomas.
Clinically, this subtype is often diagnosed at an earlier stage compared with the more common high-grade serous ovarian carcinoma, but it typically behaves more aggressively and shows resistance to standard platinum-based chemotherapy, particularly in advanced stages. As a result, the prognosis for patients with stage III or IV OCCC is generally worse than for those with high-grade serous disease.
On a molecular level, OCCC frequently carries mutations in ARID1A, PIK3CA, and PPP2R1A, while BRCA mutations and homologous recombination deficiency are much less common.
Read More about Ovarian Cancer on OncoDaily
This genetic profile explains why OCCC responds poorly to therapies such as PARP inhibitors. Current treatment usually involves surgery followed by platinum-based chemotherapy, although outcomes remain limited. Because of its resistance to standard approaches, research is now focused on new strategies, including immune checkpoint blockade, which may be particularly effective in biomarker-selected groups such as patients with PPP2R1A-mutant tumors.
You can read the full article here


